Introduction: To address the challenges associated with existing methods of ocular surface repair, Luna Innovations is developing a unique ocular biomaterial capable of protecting the injured surface and stimulating ocular healing. In this biomaterial, the healing properties of the amniotic membrane[1],[2] are mimicked in an improved hydrogel material, and the mechanical properties are enhanced using nanofiber reinforcements that effectively mimic the natural structure of the ocular tissue. The dressing production method has been developed, and standard techniques have been used to establish optical, mechanical, and biological properties.
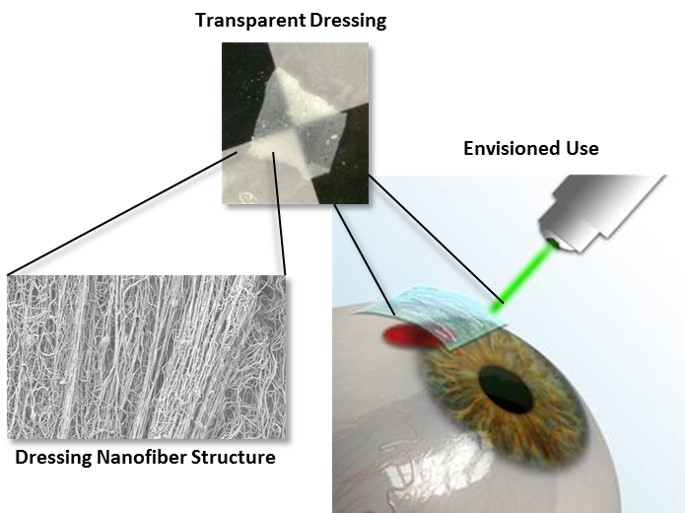
Materials and Methods: An electrospraying/spinning system for fabrication of the dressings was developed. Primary human corneal epithelial cells (HCECs) were cultured for both direct and indirect cytocompatibility testing using the lactate dehydrogenase (LDH) assay. UV-Vis spectroscopy was used to quantify transparency of the samples at 550 nm. A Vee Gee Model C10 Abbe Refractometer was used to characterize the dressing refractive index. Degradation of the construct was monitored utilizing simulated tear solution[3]. Nanofiber alignment was determined using the FibrilTool ImageJ (NIH) plugin and SEM images of the dressings[4]. Photochemical tissue bonding (dressings with 0.01 - 0.1% rose bengal, Iridix OcuLight GL laser (532 nm) at 0.25 W) was studied in vitro using excised rabbit eyeballs and in vivo using a rabbit corneal defect model[5],[6].
Results and Discussion: The goal of this study was to demonstrate the feasibility of a nanofiber-reinforced hydrogel for use as a dressing for traumatic injuries to the exposed ocular surface. Luna first assembled a unique dual electrospinning and electrospraying setup for pilot-scale production of the dressing. Cytocompatibility testing with HCECs indicated the dressings had no additional toxicity as compared to low density poly(ethylene) positive controls.UV-Vis spectroscopy indicated over 85% transmission of 550 nm light for a hydrated dressing, and the refractive index of the final dressing was found to be 1.335. Using image processing of SEM micrographs, Luna confirmed alignment of the nanofiber-reinforcing layers and demonstrated control over the degree of alignment during production, with final dressings having an anisotropy value of > 0.2. Luna also utilized 532 nm light (green) to demonstrate photochemical tissue bonding of the dressing to the surface of an excised albino rabbit eyeball. The concentration of the photoactive dye rose bengal was systematically altered in the hydrogel component of the dressing and was determined capable of adhesion to the surface with no cytotoxic effect to HCECs at 0.05% (wt/vol). Luna was able to demonstrate controlled degradation (5 minutes to 4 weeks) in simulated ocular fluids by controlling the crosslinking of the hydrogel component. Finally, Luna is implementing the dressing in a rabbit model of corneal defects to demonstrate improved healing and establish photochemical tissue bonding in vivo.
Conclusions: Luna has succeeded in demonstrating feasibility of its dressing for use as an ocular biomaterial to aid in healing of the ocular surface. The dressing was found to have a refractive index of 1.335, a transmission of 85% for 550 nm light, and to be cytocompatible with primary human corneal epithelial cells. In vivo studies are ongoing.
This material is based upon work supported by the US Army Medical Research and Materiel Command under Contract No W81XWH-14-C-0028. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.
References:
[1] Allen, C. L., et al. (2013). PloS One, 8(10), e78441.
[2] Boudaoud, A., et al. (2014). Nature Protocols, 9(2), 457–63.
[3] Dua, H. S., Gomes, J., King, A. J., & Maharajan, V. S. (2004). Survey of Ophthalmology, 49(1), 51–77.
[4] Marques, M. (2011). Dissolution Technologies, (August), 15–28.
[5] Verter, E. E., et al. (2011). Investigative Ophthalmology & Visual Science, 52(13), 9470–77
[6] Wang, Y., Kochevar, I. E., Redmond, R. W., & Yao, M. (2011). Lasers in Surgery and Medicine, 43(6), 481–89.