The functionality of the musculoskeletal system is believed to be jeopardised by glycation and the accumulation of advanced glycation end products (AGEs). Some AGEs are generated by the non-enzymatic reaction of oligosaccharides with proteins in physiological systems. In collagen rich tissues, such as tendons and ligaments, AGEs are believed to form covalent cross-links within and between collagen molecules, thereby changing the properties of the tissue. Detrimental collagen stiffening properties are believed to play a significant role in several age-related diseases such as osteoporosis[1] and cardiovascular disease[2]. This study aims to identify specific sites involved in the formation of AGE cross-links within the collagen molecule, based on a relative energetics model using a proven fully atomistic MD approach[3]. The results of this study will help to determine to what extent the position of these sites will affect the biological function and the mechanical properties of the tissue.
A dynamic distance-based criterion search was used to identify lysine and arginine residues within 5 Å of each other within a collagen molecule. Fully atomistic MD simulations, exploiting the D-band periodicity to replicate the dense fibrillar environment, were then conducted under pseudo physiological conditions in AMBER12[3]. A site is a likely candidate for glucosepane or DOGDIC formation if the total energy of the collagen molecule is lower in the presence of a cross-link compared to an unbound glucose. Using a candidate cell and matrix interaction domains map of the collagen fibril[4], we then transpose our cross-link positions to determine the biological impact of their presence. Constant velocity steered MD simulations were conducted to calculate changes in the stiffness of the collagen molecule.
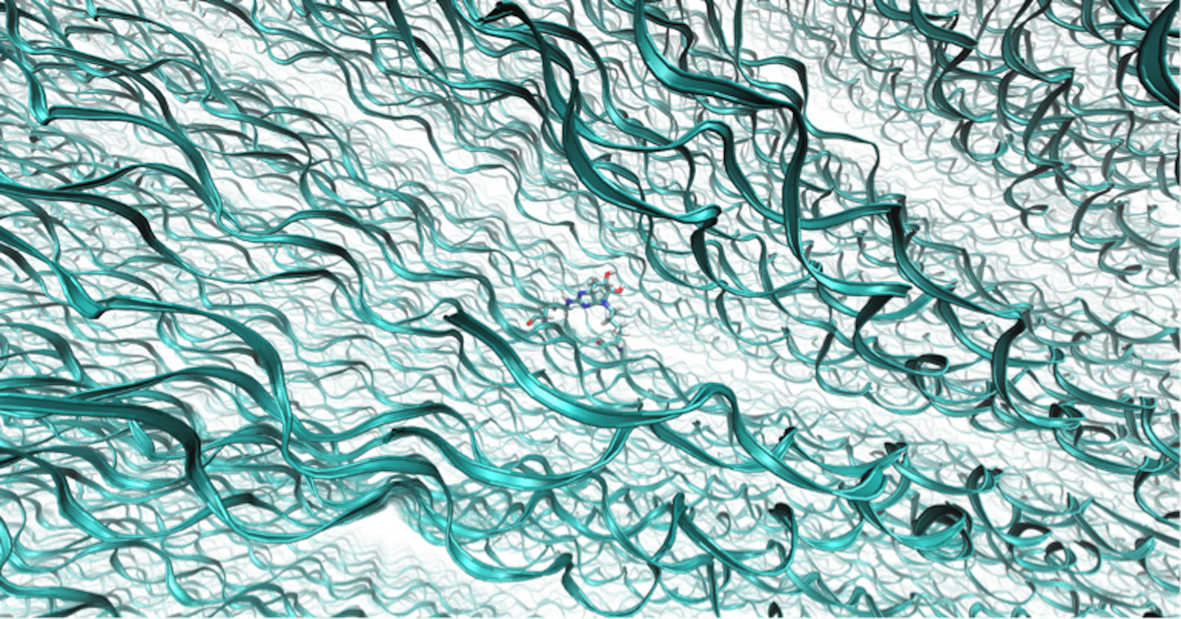
Figure 1. Dense fibrillar environment simulated within the model, with a single glucosepane molecule present at the centre of the image (Green ribbons represent collagen backbone, water and chloride ions omitted from image for clarity)
Of the 24 positions identified based on the distance criteria, 6 sites were found to be energetically favourable compared to the unbound glucose collagen model, for glucosepane and 6 for DOGDIC with only 1 duplicate site. The local environment around the site has a significant effect on the energetics, with the sites within the gap region being more likely to have favourable formation enthalpies. A number of favourable sites have potential for huge implications on the biological function of collagen, as they are within sites where key collagen-biomolecule and collagen-cell interactions occur. For example, the formation of glucosepane was found to be energetically favourable within close proximity of the Matrix Metalloproteinase-1 (MMP1) binding site, which could potentially disrupt collagen degradation. Finally the Young’s modulus was found to increase as a result of the presence of the cross-link
Our 3-dimensional collagen fibril model has identified likely sites for AGEs formation within collagen. The positioning of these sites is likely to have a significant effect on tissue function and integrity.
The authors would like to thank the BBSRC for financial support (Grant: BB/K007785)
References:
[1] X. Wang, X. Shen, X. Li, and C.M. Agrawal, “Age-related changes in the collagen network and toughness of bone.” Bone, 31, (2002), 1–7
[2] S.J. Zieman, and D.A. Kass, “Advanced glycation end product cross-linking: pathophysiologic role and therapeutic target in cardiovascular disease.” Congest. Hear. Fail. 10, (2004), 144–151
[3] I. Streeter, and N.H. de Leeuw, “Atomistic modeling of collagen proteins in their fibrillar environment.” J. Phys. Chem. B 114, (2010), 13263–13270
[4] S.M. Sweeney, J.P. Orgel, A. Fertala, J.D. McAuliffe, K.R. Turner, G.A. Di Lullo, S. Chen, O. Antipova, S. Perumal, L. Ala-Kokko, A. Forlino, W.A. Cabral, A.M. Barnes, J.C. Marini, and J.D. San Antonio, “Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates.” J. Biol. Chem. 283, (2008), 21187–21197