Encapsulation of proteins in micro- and nano-hydrogel carrier systems for controlled drug delivery
-
1
Braunschweig University of Technology, Institute for Technical Chemistry, Germany
-
2
Braunschweig University of Technology, Institute for Pharmaceutical Technology, Germany
-
3
Braunschweig University of Technology, Institute for Physical Chemistry, Germany
Introduction: Therapeutic proteins like antibodies or signaling proteins have a high potential for pharmaceutical applications. Drug delivery systems are needed to protect these proteins from in-vivo degradation. Furthermore, the proteins are able to induce unwanted side effects. A local administration close to the diseased area and a slow drug release can minimize these effects. Hydrogels provide a protein friendly environment and can have good biocompatibility. Therefore they are well suited as drug delivery systems. Diffusion and/or degradation are the main release mechanism. The relation between network structure and release was studied for bulk hydrogels and micro gels. Furthermore the in vitro and in-vivo release was studied and compared.
Materials and Methods: Hydroxyethyl starch was modified with polyethylene glycolmethacrylate HES-PEGMA, which can be photochemically cross-linked in aqueous solution in order to obtain hydrogels or injectable hydrogel microspheres. The release was investigated and correlated with the mesh size[1]. Green fluorescent protein (GFP) loaded hydrogels have been analyzed by fluorescence methods with two-photon excitation[2]. Via fluorescence anisotropy the inner rotational motions can be studied[2]. Fluorescence recovery after photo-bleaching (FRAP) gives information about the translational diffusion. In vitro release rates were compared with in vivo release rates employing fluorescently labelled proteins and an IVIS®-system[3]. Furthermore, a nanogel system prepared from chitosan and tripolyphosphate (TPP) was investigated regarding entrapment efficiency and release of signaling proteins[4].
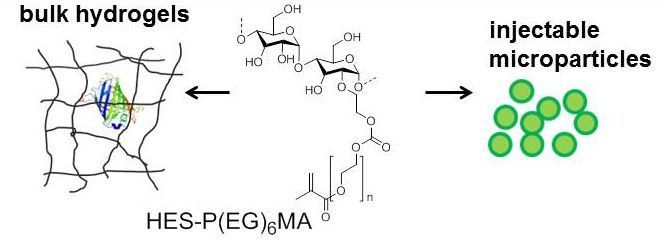
Fig.1: Chemical structure of the HES derivatives
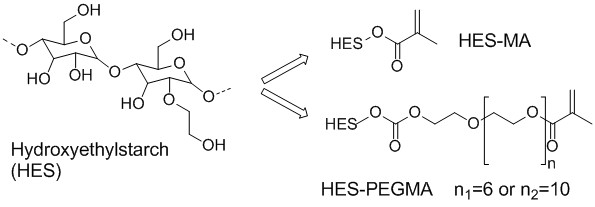
Fig. 2: Bulk hydrogels and injectable micro particles were prepared from the HES derivatives
Results and Discussion: The mesh size of the hydrogel network can be adjusted via the degree of substitution and the polymer concentration. The bulk hydrogels and the micro particles have similar structures and release profiles when prepared from the same polymer and concentration[1],[3]. The results of the fluorescence anisotropy suggest an unrestricted GFP rotation within the hydrogel, while the FRAP measurements indicate that translational mobility of GFP is restricted in the hydrogel[2]. The comparison of in vitro and in vivo release rates indicates a good comparability depending on the acceptor medium used in the in vitro experiments[3]. The chitosan/TPP nanoparticles are well suited to deliver biologically active BMP2[4].
Conclusions: Hydrogels as micro- or nanoparticles are well suited for delivery of therapeutic proteins. By adjusting the preparation methods the drug release can be tailored.
The authors would like to thank the Deutsche Forschungsgemeinschaft (DFG) for providing financial support to this project within the CRC 599 "Sustainable Bioresorbable and Permanent Implants"" and the CRC 578 “From gene to product”
References:
[1] Bertz, A. et al. J. Biotechnol. 2013, 163, 243
[2] Bertz, A. et al. Macromol. Biosci. 2013, 13, 215
[3] Wöhl-Bruhn, S. et al. J. Controlled Release 2013, 162, 127
[4] Poth, N. et al. Biomolecules 2015, 5, 3-19
Keywords:
Hydrogel,
Drug delivery,
growth factor,
Biodegradable material
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials for therapeutic delivery
Citation:
Bertz
A,
Poth
N,
Dempwolf
W,
Wöhl-Bruhn
S,
Bunjes
H,
Ehlers
J,
Gericke
K and
Menzel
H
(2016). Encapsulation of proteins in micro- and nano-hydrogel carrier systems for controlled drug delivery.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02068
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Andreas Bertz, Braunschweig University of Technology, Institute for Technical Chemistry, Braunschweig, Germany, andreas.bertz@gmx.net
Dr. Nils Poth, Braunschweig University of Technology, Institute for Technical Chemistry, Braunschweig, Germany, nils.poth@gmail.com
Dr. Wibke Dempwolf, Braunschweig University of Technology, Institute for Technical Chemistry, Braunschweig, Germany, w.dempwolf@tu-bs.de
Dr. Stefanie Wöhl-Bruhn, Braunschweig University of Technology, Institute for Pharmaceutical Technology, Braunschweig, Germany, Email1
Dr. Heike Bunjes, Braunschweig University of Technology, Institute for Pharmaceutical Technology, Braunschweig, Germany, heike.bunjes@tu-bs.de
Dr. Jan-Eric Ehlers, Braunschweig University of Technology, Institute for Physical Chemistry, Braunschweig, Germany, Email2
Dr. Karl-Heinz Gericke, Braunschweig University of Technology, Institute for Physical Chemistry, Braunschweig, Germany, k.gericke@tu-bs.de
Dr. Henning Menzel, Braunschweig University of Technology, Institute for Technical Chemistry, Braunschweig, Germany, Email3