Introduction: Through engineering polymer and nanoparticle properties, non-viral intracellular delivery of DNA and siRNA can be made efficacious. By delivering DNA in conjunction with inorganic nanoparticles, such as biocompatible gold nanoparticles, therapeutic function (gene/thermotherapy) can be combined with diagnostic function (imaging).
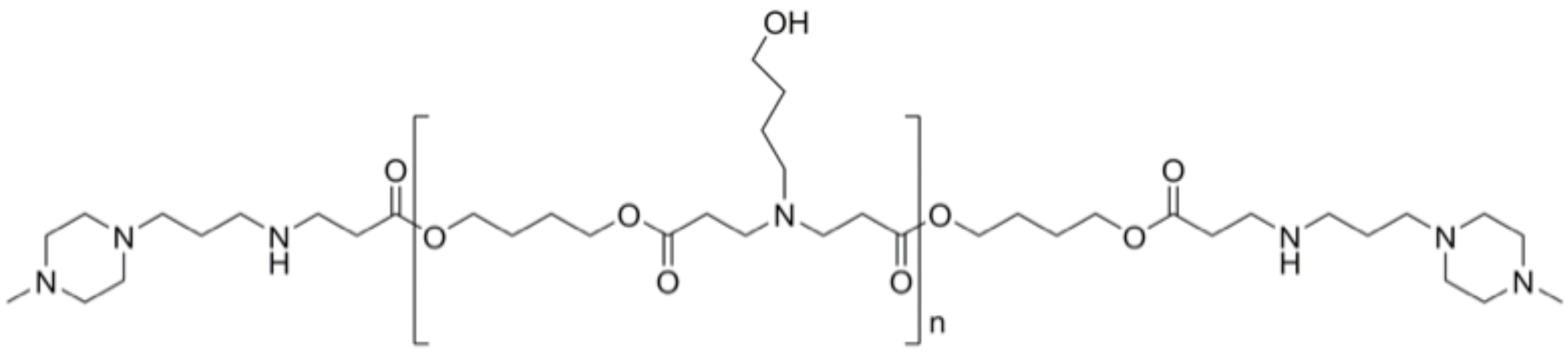
Figure 1. Representative PBAE polymer 447
Materials and Methods: Libraries of poly(beta-amino esters) (PBAEs) were synthesized as described[1]. As an example, to synthesize polymer 447 (Figure 1), 1,4-butanediol diacrylate and 4-amino-1-butanol were reacted neat in a 1.2:1 molar ratio for 24 hrs. Subsequently, the linear polymers were end-capped with 1-(3-aminopropyl)-4-methylpiperazine in anhydrous tetrahydrofuran (THF) for 1 hour at 1000 RPM. Polymeric nanoparticles were formed by combining cationic PBAEs with anionic DNA or siRNA and allowing for self-assembly. Gold nanoparticles (AuNPs) were synthesized via a modified Frens method. Multilayer theranostic nanoparticles were designed following Figure 2. Fluorescence microscopy and flow cytometry were performed over time to measure transfection efficacy of reporter genes in GB319 human glioblastoma cells. siRNA-mediated gene knockdown was performed in constitutively eGFP positive GB319s. Cellular viability was measured by the CellTiter96® assay. In vivo evaluation of non-viral gene delivery nanoparticles was performed in an orthotopic patient-derived glioblastoma model in male nude athymic mice[2].
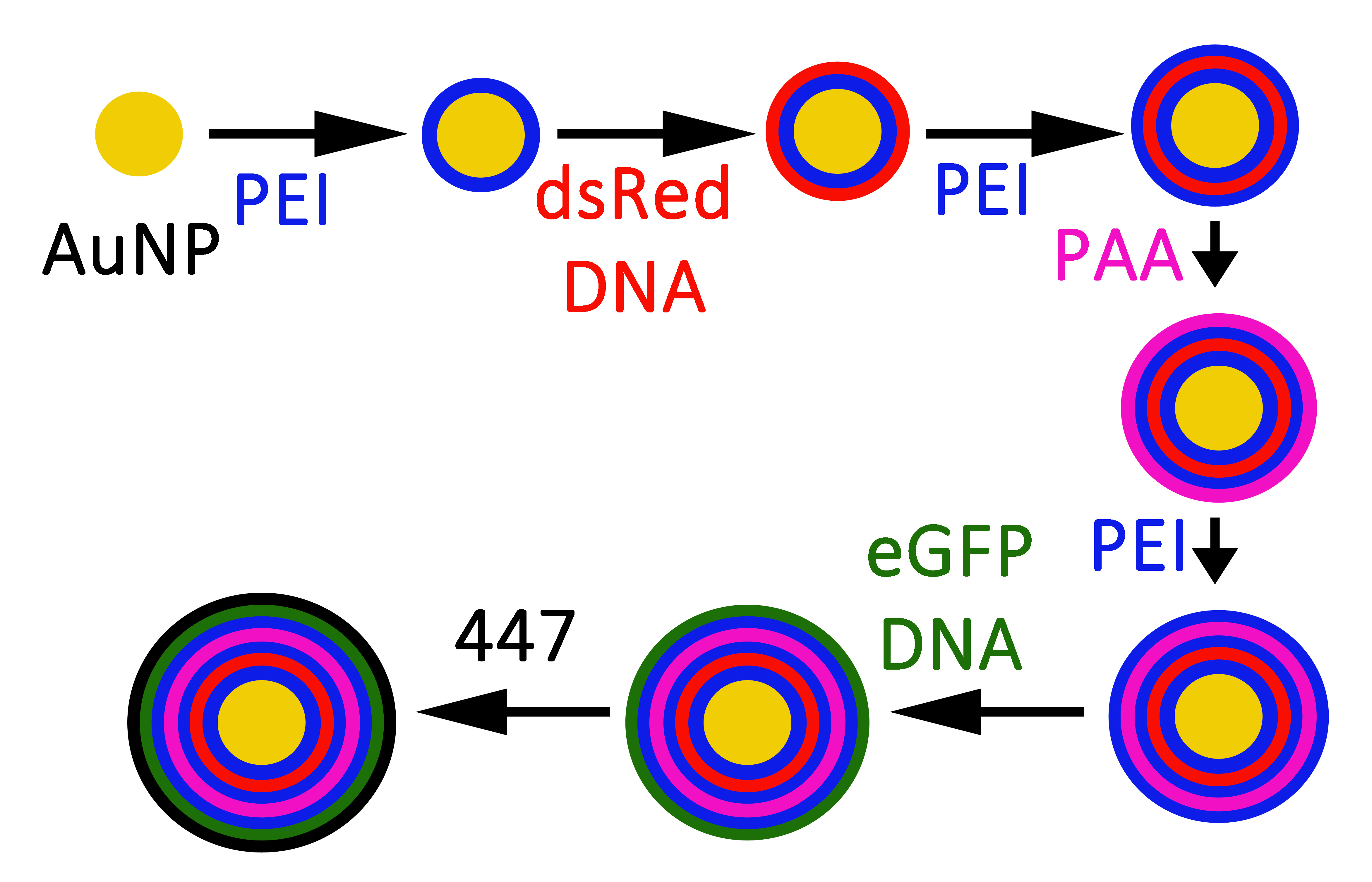
Figure 2. The layer-by-layer (LbL) fabrication process to create inorganic/polymeric hybrid nanoparticles.
Results and Discussion: Non-bioreducible PBAE nanoparticles were found to be highly effective for DNA delivery, reaching 90% transfection efficacy without causing non-specific cytotoxicity. Certain bioreducible PBAE nanoparticles were found to be relatively ineffective for DNA delivery, but highly effective for siRNA delivery. These bioreducible nanoparticles led to 90% gene knockdown also without causing non-specific cytotoxicity. Polymers with increased hydrophobicity were found to be more effective for both DNA and siRNA delivery. When administered in vivo, 447/DNA nanoparticles successfully transfected the patient-derived glioblastoma cells in the mouse model and also demonstrated tumor-cell specific transfection. To enable kinetic control of exogenous gene expression of multiple genes, layer-by-layer (LbL) inorganic/polymeric hybrid nanoparticles were successfully fabricated. The zeta potential of the LbL inorganic/polymeric nanoparticles reversed at each of the 7 stages of layering, confirming that the coatings of each polyelectrolyte layer were achieved. The outer eGFP DNA layer of the LbL inorganic/polymeric nanoparticles transfected highly at day 2 and this level of gene expression persisted until day 9, when it then decreased over time (Figure 3). In contrast, the inner dsRed DNA layer had low expression initially that steadily increased to a maximum at day 9. Temporal control of multi-gene delivery was achieved by the physical placement of these different plasmids within the LbL layers and by biomaterial properties.
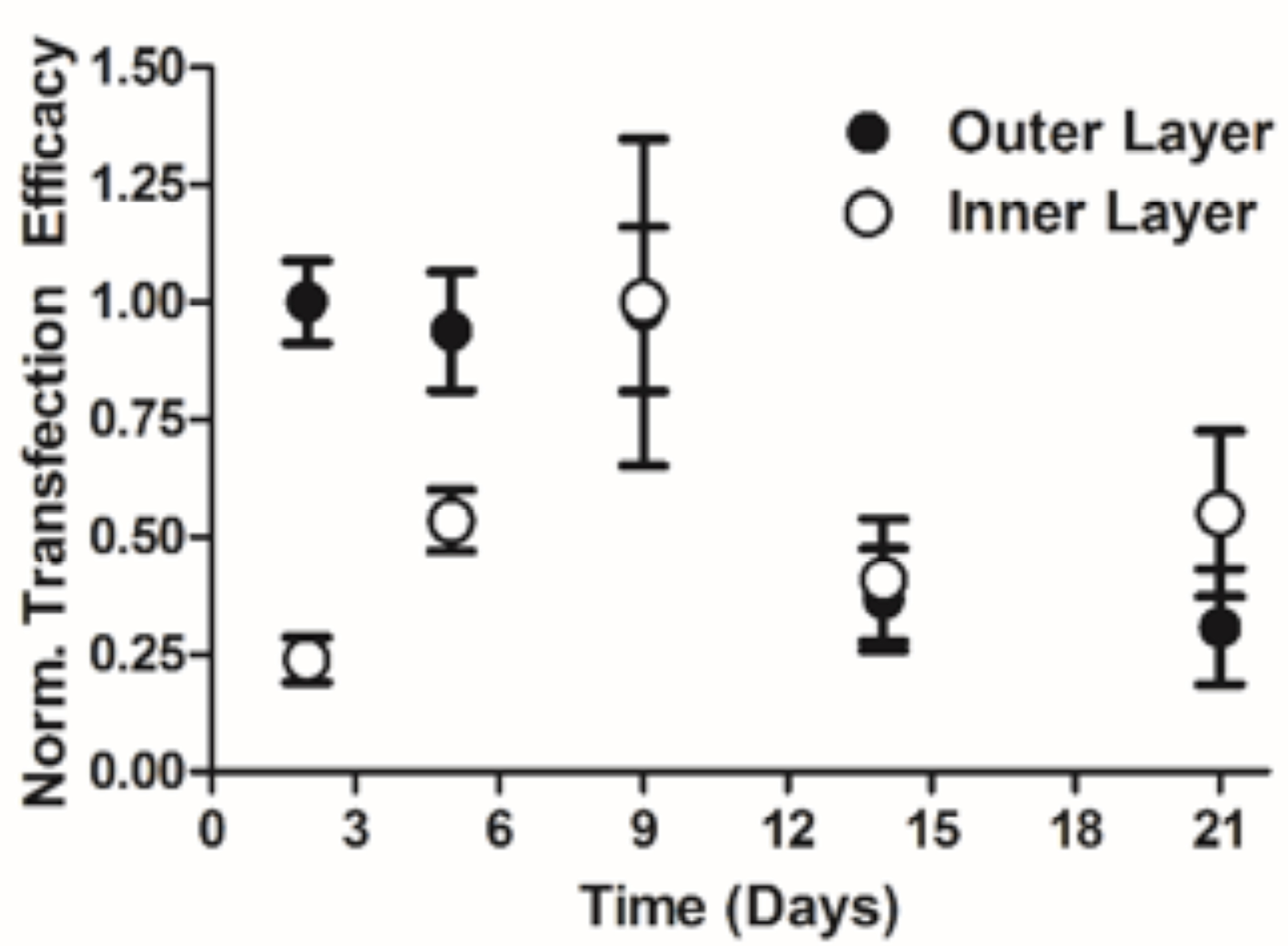
Figure 3. Normalized transfection efficacies of inorganic/polymeric hybrid nanoparticles demonstrating temporal control of exogenous gene expression in human glioblastoma cells.
Conclusion: Using a polymer library approach we were able to achieve high non-viral DNA delivery with low cytotoxicity as well as high siRNA knockdown with low cytotoxicity to human brain cancer cells. Certain polymer features, including hydrophobicity and mode of degradation, were key parameters for the effective nanomaterials. In addition, these nanoparticles showed efficacy in vivo in a human glioblastoma mouse model. Using an LbL approach, we were able to successfully develop a method to electrostatically coat inorganic theranostic nanoparticles that can load two different types of plasmids and deliver them with temporal control.
National Institutes of Health (R01EB016721)
References:
[1] Tzeng SY, Green JJ. Subtle changes to polymer structure and degradation mechanism enable highly effective nanoparticles for siRNA and DNA delivery to human brain cancer. Adv Healthc Mater 2013;2(3):468-80.
[2] Guerrero-Cazares H, Tzeng SY, Young NP, Abutaleb AO, Quinones-Hinojosa A, Green JJ. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano 2014;8(5):5141-53.