Introduction: Mechanical stimuli play a role on tissue formation and mineralization capabilities of cells[1],[2]. Here, we propose a novel 3D composite polymeric scaffold (PCL) embedded with collagen gel as support for tissue growth to investigate the effect of compression on hMSCs activities. Collagen degradation and tissue development are quantified by employing Micro Computed Tomography (MicroCT) in order to demonstrate the effectiveness of compression on bone tissue formation in internal volumes of 3D composite scaffolds closely mimicking bone architecture.
Materials and Methods: Collagen/hMSCs injection. 3D Insert® PCL (3D Biotek, USA) underwent air plasma treatment (5 min, 30W, 1 mBar) and sterilization by 70% ethanol. hMSCs (20*10^3 cells/sample) were suspended in 2 mg/ml bovine type 1 collagen and seeded placing 40 µl volume on samples. Collagen solidifies after 1.5 h incubation.
Mechanical stimulation. From day 6, samples underwent compression applying a 5% ramp followed by peak-to-peak 1% sinewaves at 1 Hz for 15 min. Three different conditions were tested considering unloaded (U), loaded from day 6 to day 10 (L1) and loaded as L1 and from day 16 to day 20 (L2).
Assays and MicroCT. At day 1, 3, 7, 14, 21 and 28, samples were collected for testing cells viability by Presto Blue assay, DNA and osteocalcin quantification. Samples were stained with 1% osmium tetroxide, scanned by MicroCT (40KV, 255uA, 10W and pixel resolution 17.4 μm) and reconstructed using Simpleware. Experiments were repeated three times for statistical analysis.
Results and Discussions: Proliferation becomes significant in L1 conditions from day 21 and on U samples at day 14 (p<0.05) while comparable DNA amount is detected in L2 conditions.
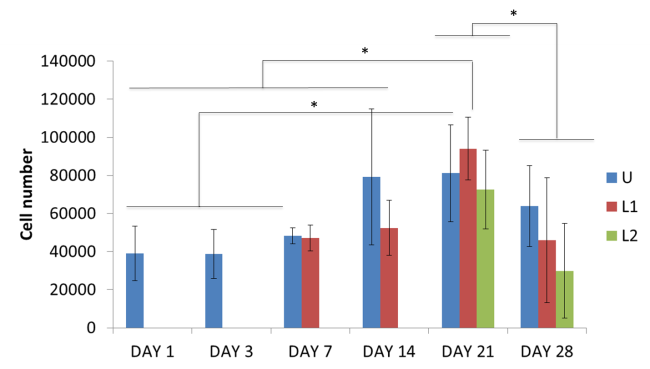
These outcomes highlight the negative effect elicited by compressions stimuli on cell proliferation. In all cases, a drop in DNA content is noticeable at day 28 as a consequence of the formation of an external cellular layer preventing diffusion of nutrients in the internal volume of the construct and progressively causing cell apoptosis and death. At day 28, MicroCT reveals higher tissue content while ELISA shows enhanced osteocalcin production for L2 samples compared to other conditions at day 28, confirming mineralization to occur. Presto Blue results show a diverse trend with higher metabolic activity for all conditions tested referring to the cellular layer developing on samples surface.
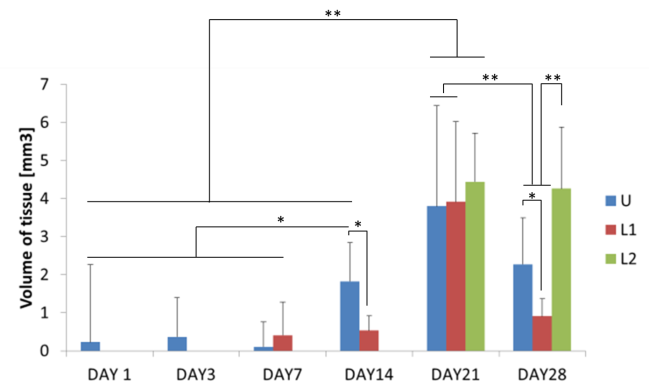
Conclusions: This study shows how short periods of compression can be an effective tool to modify cellular activities. Compression is shown to affect tissue formation and mineralization potential of hMSCs over time depending on the application time. MicroCT demonstrates to be a promising tool to monitor tissue and mineral formation, opening the frontiers to a new approach focused on the investigation of cellular behaviour into opaque 3D starches.
European Research Council (FP7-258321)
References:
[1] A. Sittichockechaiwut, A. M. Scutt, A. J. Ryan, L. F. Bonewald, and G. C. Reilly, Bone, vol. 44, no. 5, pp. 822–9, May 2009.
[2] N. J. Steinmetz and S. J. Bryant, Acta Biomater., vol. 7, no. 11, pp. 3829–40, Nov. 2011.