Mesoporous silica nanoparticles: Particle size control for optimal controlled drug release and unique therapeutic delivery
-
1
axe Médecine Régénératrice, Centre de recherche du Centre hospitalier universitaire de Québec (CR-CHUQ), Canada
-
2
axe Cancer, Centre de recherche du Centre hospitalier universitaire de Québec (CR-CHUQ), Canada
-
3
Université Laval, Department of Mining, Metallurgy and Materials Engineering, Canada
-
4
Université Laval, Department of Chemistry, Canada
Mesoporous silica nanoparticles (MSN) have emerged as promising materials for drug delivery applications due to their unique porosity and surface characteristics. Conventional MSN have a particle size between 100 – 150 nm. The cellular uptake, tissue accumulation and blood circulation half-life of these particles are always limited due to their large size. Sub-100 nm MSN are needed to overcome these limitations and ameliorate their therapeutic delivery performances. In this study, we report how surface functionalization and particle size control of MSNs could ensure the design of nanoparticles with high drug loading, efficient in vitro and in vivo controlled drug release and unique therapeutic delivery.
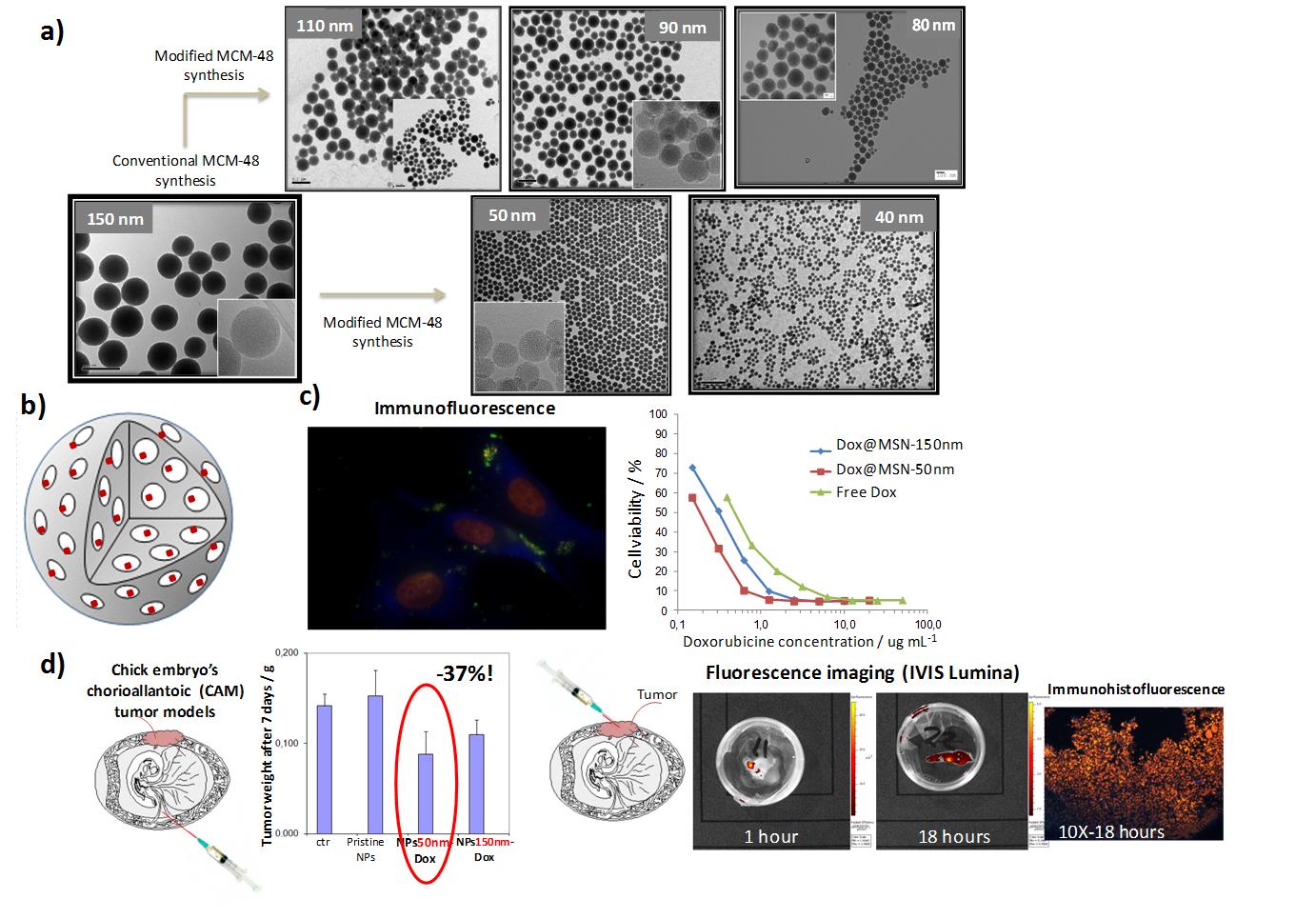
Therefore, size-controllable MSN (from 40 to 150 nm) were synthesized and functionalized with phosphonate groups (MSN-P) in order to increase the electronegativity of the surface charge (for better interaction with doxorubicin, a positively charged anticancer molecule) and to improve the colloidal stability and the bio and hemocompatibility of MSN. All obtained nanoparticles were thoroughly characterized by TGA, NMR, N2 physisorption, TEM and XPS analyses. Colloidal stability was investigated in different physiological media and monitored by dynamic light scattering. Biocompatibility was evaluated by standard cell viability and cell proliferation assays. Drug loading assays were performed by using doxorubicin (an anticancer molecule) in aqueous solution. Drug release efficiency as well as accumulation and diffusion into tumors were investigated in physiological conditions (neutral and acidic buffer solutions: PBS pH 7,4 and Phosphonate buffer pH = 5), in vitro with human cancer cell lines (M21 and HT1080) and in vivo using chick embryo’s chorioallantoic tumor model.
Pure and functionalized MSN were colloidally stable in saline, simulated body fluid and cell culture media, without any evidence of cell toxicity. Drug loading capacities up to 6 times more than reported in the literature, were observed with functionalized MSN (MSN-P), regardless of the particle size. For drug elution properties, an efficient pH-dependent and size-dependent release of doxorubicin was achieved in physiological acidic conditions, as demonstrated by the pharmacokinetic profiles (the highest release rate of doxorubicin was obtained with Sub-100 nm MSN). The effects of particle size on the drug release (kinetic study) were also visualized in the intracellular compartments and into the administered tumors. In fact, an efficient cellular uptake, tumor accumulation and drug diffusion was observed with small particles ≤ 50 nm (ex vivo fluorescence imaging and immuno/immunohistofluorescence). Furthermore, strong impact on cell and tumor growth inhibition was observed with these small particle size (e. g. tumor growth inhibition was around 40% with Dox@MSN-50nm vs only 25% with Dox@MSN-150nm after only 7 days of nanoparticles intravenous injection).
We demonstrated thereby the strong potential of small MSN (≤ 50 nm diameter) as drug-elution nanoparticles compared with conventional larger MSN (e.g. 150 nm diameter). This represents a significant and original step toward the development of tailored nanobiomaterials as a next-generation of theranostic carriers for drug delivery and imaging applications.
Fonds québécois de la recherche sur la nature et les technologies (FRQNT); Dr Pascale Chevalier
Keywords:
Drug delivery,
nanoparticle,
biomedical application,
targeting delivery
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials for therapeutic delivery
Citation:
Bouchoucha
M,
Côté
M,
Gaudreault
RC,
Fortin
M and
Kleitz
F
(2016). Mesoporous silica nanoparticles: Particle size control for optimal controlled drug release and unique therapeutic delivery.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02085
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Meryem Bouchoucha, axe Médecine Régénératrice, Centre de recherche du Centre hospitalier universitaire de Québec (CR-CHUQ), Québec, QC, Canada, Email1