Introduction: Selective labeling and identification of receptors on cells can provide important clinical information, such as distinction between healthy and diseased cells, early detection and evolution of a disease, patient-specific drug selection and monitoring of the therapeutic response[1]. Immunofluorescence is the gold standard for efficient detection of antigens expressed by cells. Antibodies (Abs) conjugated to fluorescent dyes are mainly developed in the visible wavelengths and remain limited by photobleaching, high sensitivity to the environment, low light intensity, and wide absorption and emission spectra[2].
Tunable plasmonic nanoparticles (NPs) should provide higher multiplexing capacity than immunofluorescence since NPs are photostable over time, emit high light scattering at a specific wavelength (plasmon peak) and can be synthesized and functionalized with Abs[3]-[6]. The scattering peaks of silver (Ag) and gold (Au) nanospheres (NSs) are around 450 and 550 nm, respectively, and the ones from Au nanorods (AuNRs) can be extended from 600 to 2200 nm[7]. Microscopy at various wavelengths allows low illumination and fast integration times for spectral characterization of NPs in cellular environment[8]-[12]. We aim to use reflected light microscopy (RLM) for three-dimensional (3D) wide-field imaging of Abs-functionalized NPs (immunoplasmonics fNPs) targeting cell surface receptors as an alternative to immunofluorescence.
Materials: Abs anti-CD44 and anti-EGFR from abcam and Abs anti-KV1.1 from Alomone Labs. Orthopyridyl-disulfide-poly(ethylene glycol) (5kDa)-N-hydroxysuccinimide (OPSS-PEG-NHS) and HS-PEG (5kDa) from Nanocs. 80 nm AgNSs from Ted Pella. 100 nm AuNSs and 40 nm x 92 nm AuNRs from Nanopartz. PBS and DAPI from Sigma-Aldrich. Secondary Abs (Alexa 488 and Cy3) from Life Technologies.
Methods: Abs were conjugated to OPSS-PEG-NHS (OPSS-PEG-Ab)[10],[13]. Citrate-capped NPs were functionalized with 0.01 OPSS-PEG-Ab/nm2 and 5 µM HS-PEG (5kDa): CD44-AgNSs, EGFR-AuNSs and KV1.1-AuNRs. The stability of fNPs was confirmed by UV-vis spectroscopy. The expression of CD44, EGFR and KV1.1 receptors was detected by immunofluorescence and RLM on MDA-MB-231 breast cancer cells and on 661W photoreceptors. Cells were incubated for 3 h with 8 μg/mL fNPs, washed with PBS and fixed. RLM system for 3D wide-field imaging was built on an inverted Eclipse Ti microscope (Nikon) with spectral filters (500, 580 and 700 nm, Thorlabs) for fast z-scanning, optimal spectral separation and optical contrast of the fNPs in cellular environment (Fig. 1).
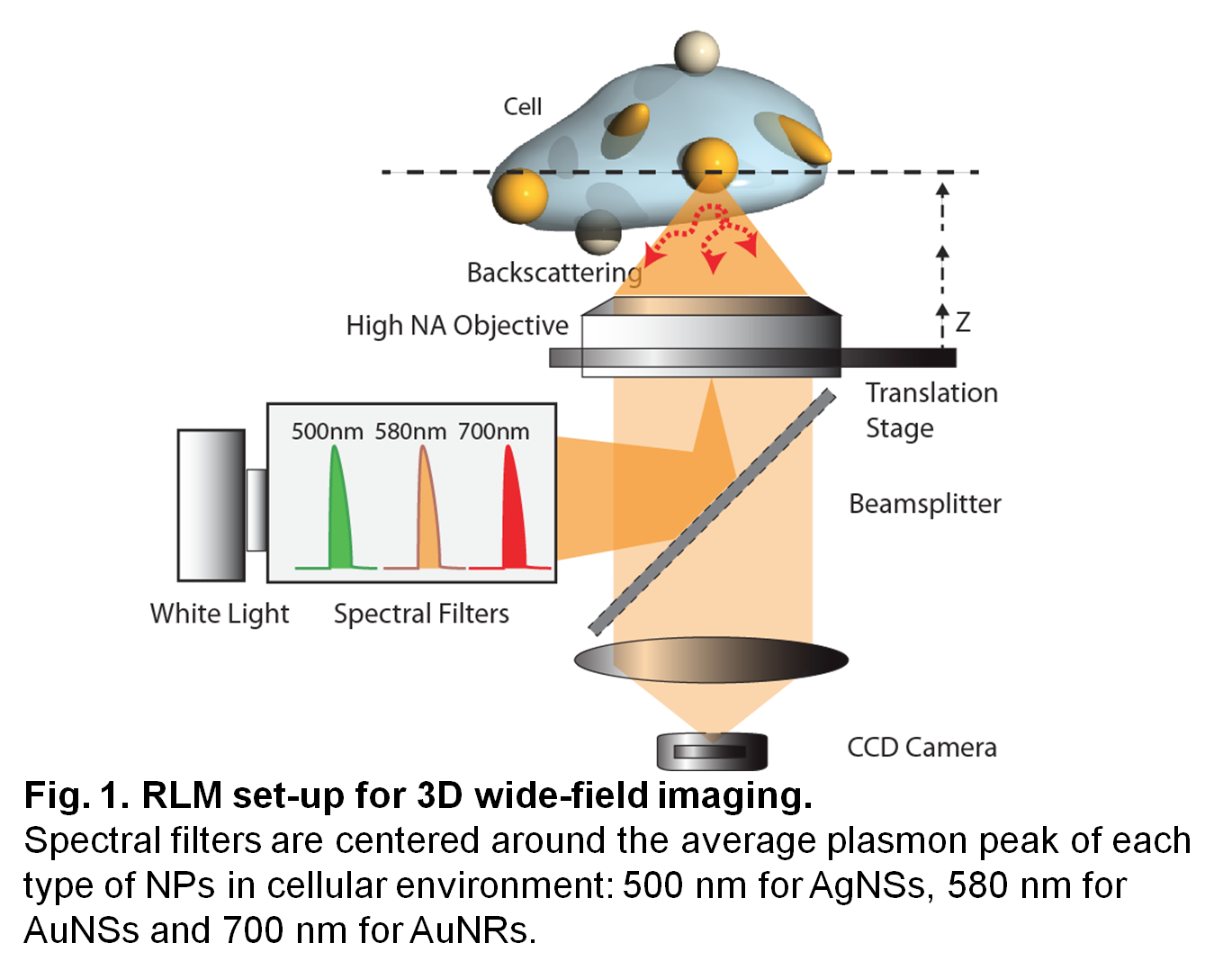
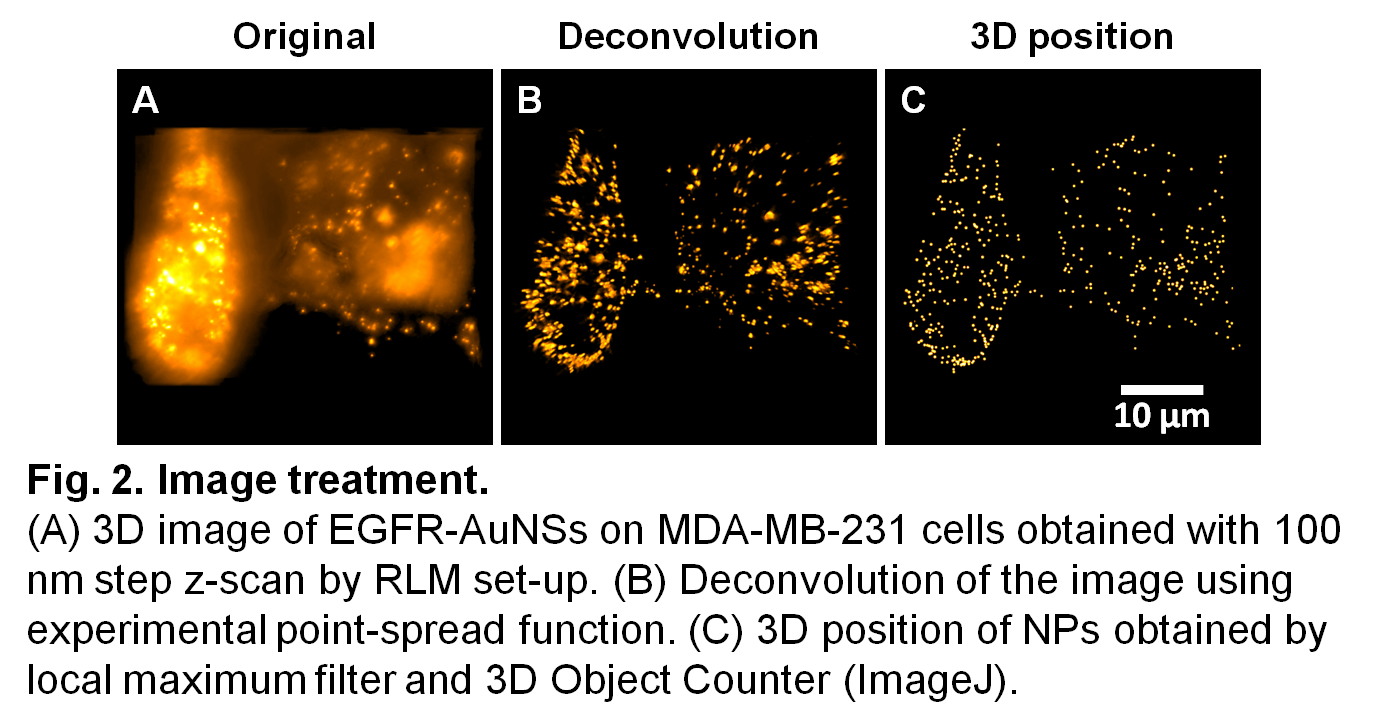
Results and discussion: Deconvolution was applied to each image and treated with ImageJ to generate an image taking into account the average plasmon peak of each type of NP (Fig. 2).
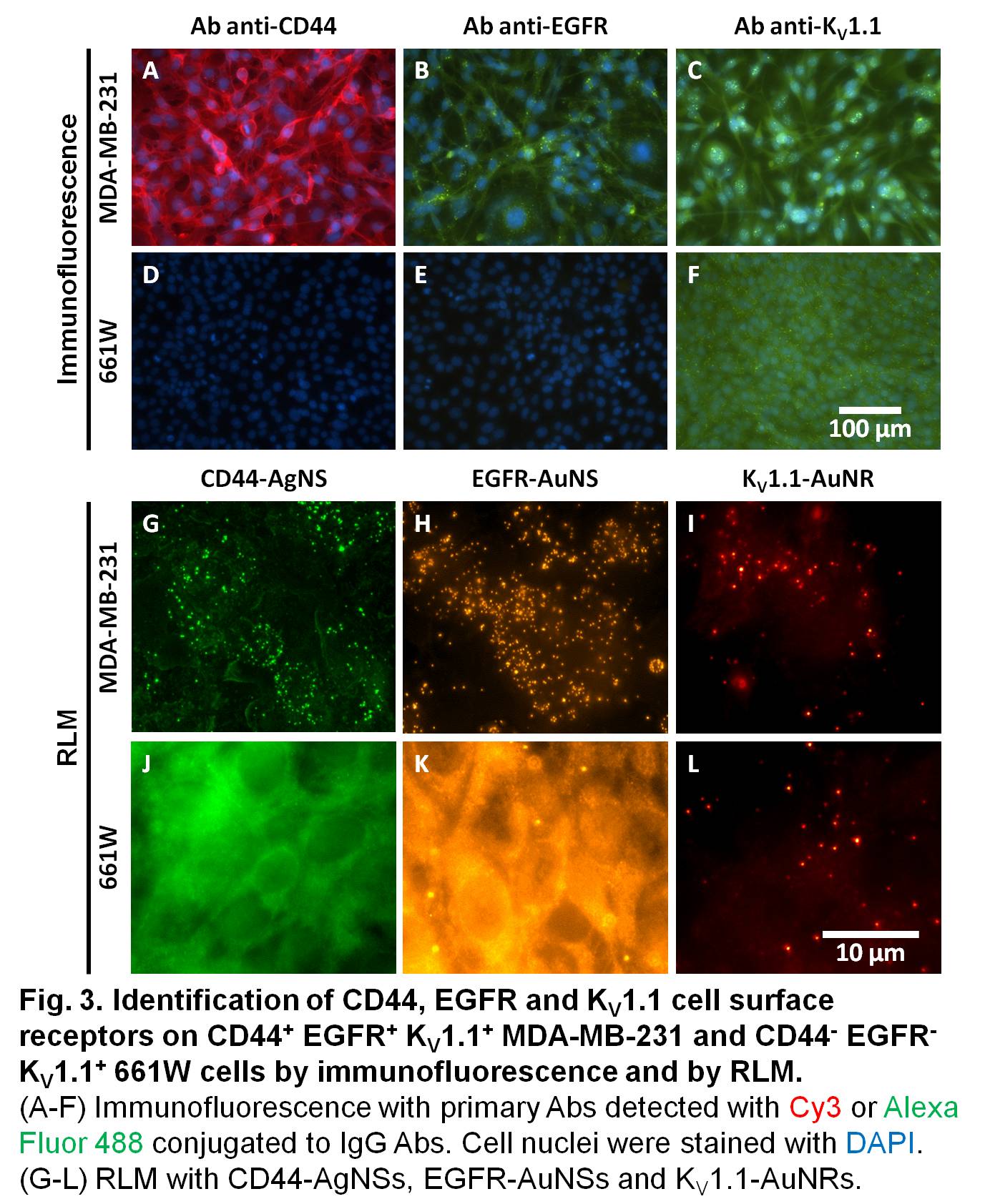
Immunofluorescence demonstrated the expression levels of targeted receptors (Fig. 3A-F): CD44+ EGFR+ KV1.1+ MDA-MB-231 and CD44- EGFR- KV1.1+ 661W. The exposure time to detect CD44 was 80 ms while it was longer for EGFR and KV1.1 (500 ms). By increasing the exposure time, the background fluorescence and photobleaching become more important. This problem is solved with improved 3D identification of stable fNPs selectively labeling targeted cells by RLM (Fig. 3G-L).
Conclusion: The developed technology is simple and compatible with standard fluorescence microscopy set-up. This technology with optical analysis of biomarkers is ready for clinical applications as an alternative to immunofluorescence.
This work was supported by Le Fonds de recherche du Québec and the Natural Science and Engineering Research Council of Canada. EB received funding from Fonds de recherche du Québec – Santé. Dr. Laudine Desreumaux-Communal and Prof. Anne-Marie Mes-Masson from CRCHUM are acknowledged for fruitful discussions.
References:
[1] J. Aaron, N. Nitin, K. Travis, S. Kumar, T. Collier, S. Y. Park, M. José-Yacamán, L. Coghlan, M. Follen, R. Richards-Kortum and K. Sokolov (2007) Plasmon resonance coupling of metal nanoparticles for molecular imaging of carcinogenesis in vivo, Journal of Biomedical Optics 12(3):034007.
[2] P. Zhang, S. Lee, H. Yu, N. Fang and S. H. Kang (2015) Super-resolution of fluorescence-free plasmonic nanoparticles using enhanced dark-field illumination based on wavelength-modulation, Scientific Reports 5:11447.
[3] X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed (2007) Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy, Nanomedicine 2(5):681−693.
[4] K. Weintraub (2013) The new gold standard, Nature 495(7440):S14−S16.
[5] D. Rioux and M. Meunier (2014) Alloy nanoparticles, process for their preparation and use thereof, USPTO61945276.
[6] S. Patskovsky, E. Bergeron, D. Rioux, M. Simard and M. Meunier (2014) Hyperspectral reflected light microscopy of plasmonic Au/Ag alloy nanoparticles incubated as multiplex chromatic biomarkers with cancer cells, Analyst 139(20):5247–5253.
[7] K. Seekell, M. J. Crow, S. Marinakos, J. Ostrander, A. Chilkoti and A. Wax (2011) Hyperspectral molecular imaging of multiple receptors using immunolabeled plasmonic nanoparticles, Journal of Biomedical Optics 16(11):116003.
[8] N. Fairbairn, A. Christofidou, A. G. Kanaras, T. A. Newman and O. L. Muskens (2013) Hyperspectral darkfield microscopy of single hollow gold nanoparticles for biomedical applications, Physical Chemistry Chemical Physics 15(12):4163−4168.
[9] S. Patskovsky, E. Bergeron and M. Meunier (2015) Hyperspectral darkfield microscopy of PEGylated gold nanoparticles targeting CD44-expressing cancer cells, Journal of Biophotonics 8(1–2):162–167.
[10] S. Patskovsky, E. Bergeron, D. Rioux and M. Meunier (2015) Wide-field hyperspectral 3D imaging of functionalized gold nanoparticles targeting cancer cells by reflected light microscopy, Journal of Biophotonics 8(5):401–407.
[11] S. Patskovsky, D. Rioux, M. Meunier and E. Bergeron (2014) A method for imaging a sample incorporating plasmonic nanoparticles and device therefore, USPTO61987274.
[12] H. Wang, G. Rong, B. Yan, L. Yang and B. M. Reinhard (2011) Optical sizing of immunolabel clusters through multispectral plasmon coupling microscopy, Nano Letters 11(2):498−504.
[13] E. Bergeron, C. Boutopoulos, R. Martel, A. Torres, C. Rodriguez, J. Niskanen, J.-J. Lebrun, F. M. Winnik, P. Sapieha and M. Meunier (2015) Cell-specific optoporation with near-infrared ultrafast laser and functionalized gold nanoparticles, Nanoscale (in revision).