Introduction: Cell-based tissue engineering strategies for tendon repair have limited clinical applicability due to delayed extracellular matrix (ECM) deposition and subsequent prolonged culture periods, which lead to tenogenic phenotypic drift. Deposition of ECM in vitro can be enhanced by macromolecular crowding (MMC), a biophysical phenomenon that governs the intra- and extra-cellular milieu of multicellular organisms[1],[2], which has been described to accelerate ECM deposition in human tenocytes[1]. A variety of cell sources have been studied for tendon repair including tenocytes, dermal fibroblasts and mesenchymal stem cells (MSCs)[3] and various biophysical, biochemical and biological tools have been used to mimic tendon microenvironment and induce phenotype maintenance in long term cultures or differentiation[4]. Therefore, we propose to assess the combined effect of macromolecular crowding and mechanical loading on different cell sources to determine their suitability for the in vitro fabrication of tendon-like tissue.
Materials and Methods: Human dermal fibroblasts, tenocytes and bone marrow mesenchymal stem cells were cultured for 3 days with 100 µg/ml of carrageenan (MMC) under static and dynamic culture conditions. Cyclic uniaxial strain was applied using a MechanoCulture FX (CellScale) at 1 Hz and 10% strain for 12 hours a day. Cell morphology and alignment were evaluated by fluorescein isothiocyanate (FITC) labelled phalloidin and 4’,6-diamidino-2-phenylindole (DAPI) staining. Extracellular matrix composition was evaluated by immunocytochemistry. Cell phenotype maintenance/differentiation (tenogenic, chondrogenic and osteogenic lineages) were assessed by gene and protein analysis.
Results and Discussion: After 12 hours of exposure to the uniaxial load, cells are strictly aligned in the direction perpendicular to the load, as seen in Figure 1 for the dermal fibroblasts. Similar behaviour was seen for tenocytes and MSCs. ECM deposition is increased in the presence of MMC and this effect is maintained under mechanical loading. On Figure 2 it can be seen an increase in deposition of collagen I for all different cell types. Other ECM molecules have been assessed and a similar behaviour was observed. Markers for osteogenic and chondrogenic lineage have been assessed and were shown not to be expressed.
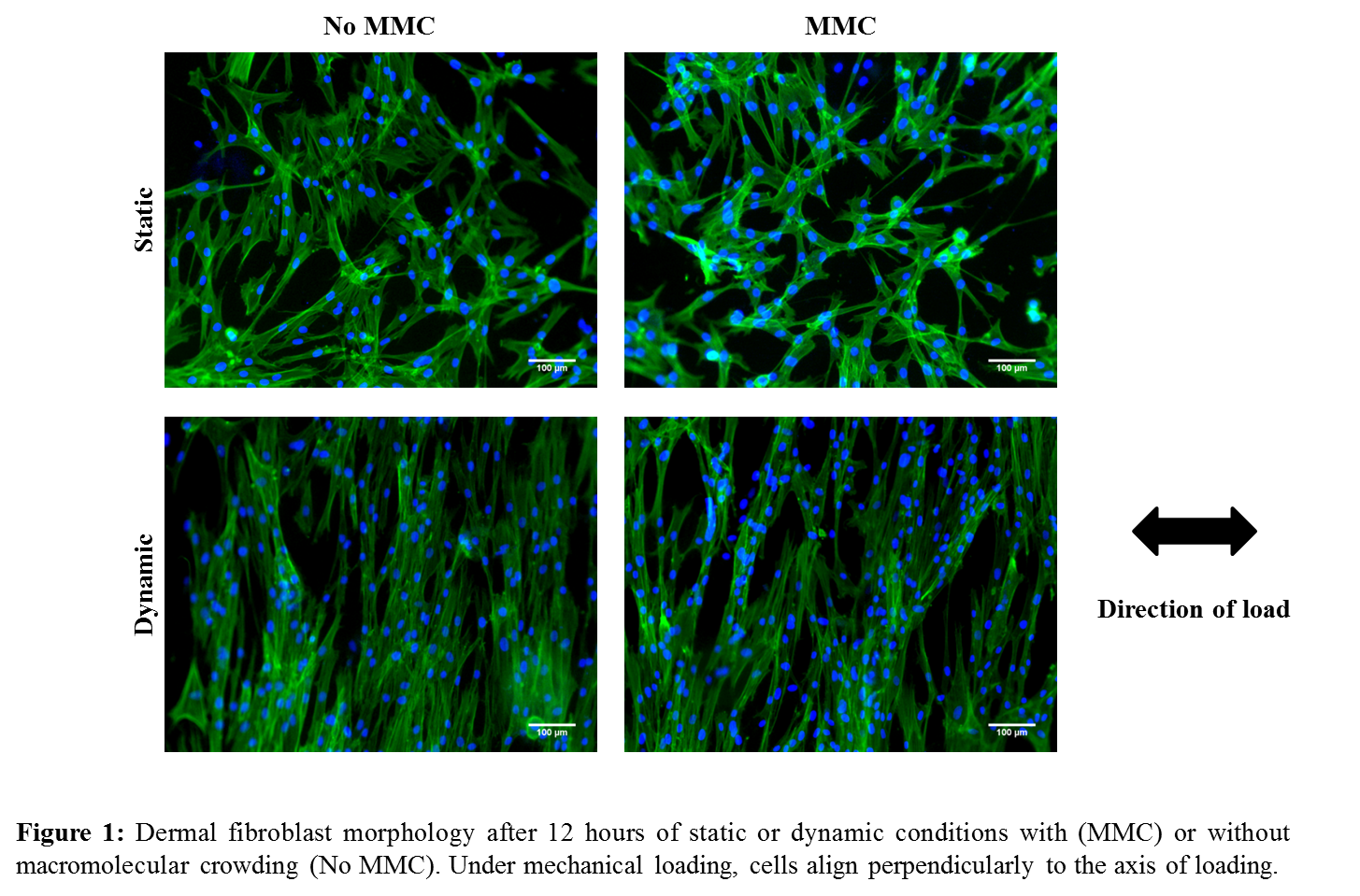

Conclusion: Mechanical loading and macromolecular crowding can induce cell and ECM alignment and increased ECM deposition without affecting cell metabolic activity or viability. ECM composition and tenogenic marker expression suggest this approach might be suitable to maintain or differentiate towards tenogenic lineage.
Irish Research Council
References:
[1] Satyam A. et al, Adv Mat, 26:3024-3034, 2014
[2] Chen C. et al, Adv Dr Del Rev 63, 277-290, 2011
[3] Gaspar D. et al, Adv Drug Deliv Rev, 84, 240-256, 2015
[4] Spanoudes K. et al, Trends in Biotech,32, 474-482, 2014