Introduction: Recently, several “smart” biomaterials that respond to specific stimuli have been studied to deliver triggered high/low or on/off doses of a drug locally inside the body, which has proven difficult to achieve in a non-invasive manner[1]. In response, we have fabricated nanocomposite materials comprised of thermosensitive microgels and superparamagnetic iron oxide nanoparticles (SPIONs), which heat in response to an alternating magnetic field (AMF), entrapped within an injectable hydrogel matrix. Upon AMF application, the SPIONs will generate heat that is transferred to the thermosensitive microgels within the nanocomposite, causing them to deswell and open up free volume that encourages drug release (Fig. 1). Relative to earlier systems that included SPIONs within a thermosensitive matrix, in which AMF-controlled release was too small and short-lived for practical use[2], the free volume generated here by the microgels deswelling greatly increases both the magnitude and duration of drug pulses that can be achieved.
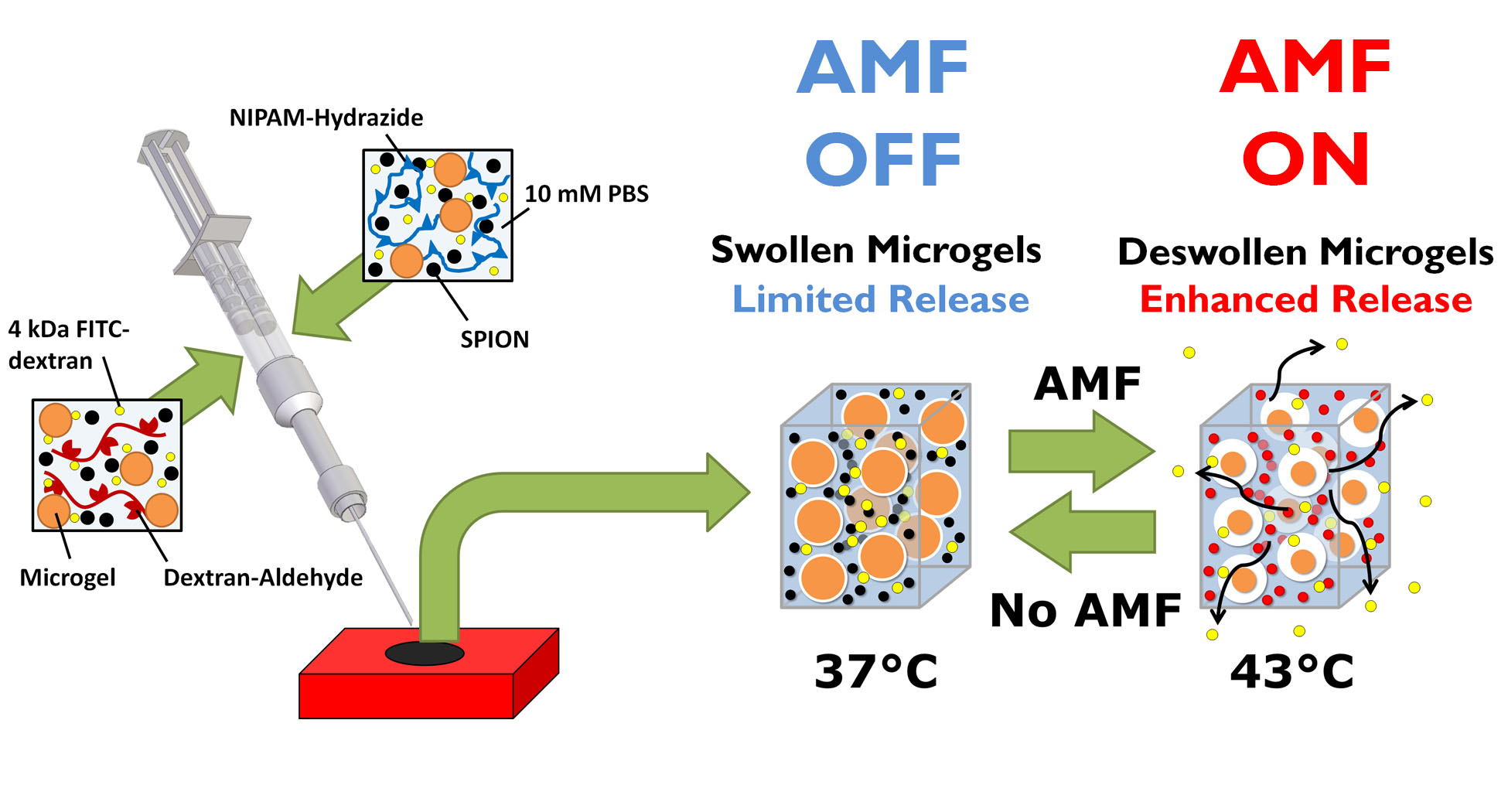
Figure 1: Fabrication and release mechanism of nanocomposite hydrogels.
Materials and Methods: SPIONs were produced by coprecipitation and peptized with poly(ethylene glycol). Microgels were fabricated using the conventional precipitation-emulsion approach via copolymerization of N-isopropylacrylamide (NIPAM) and N-isopropylmethacrylamide. Bulk injectable hydrogels are formed via the reaction of hydrazide-functionalized pNIPAM and aldehyde-functionalized dextran. Nanocomposites were formed by mixing SPIONs, microgels, and the model drug (4 kDa FITC-dextran) with the injectable hydrogel precursor polymers and coextruding through a 2-barreled syringe (Fig. 1). A specialized AMF setup was used to determine the degree of control over release over multiple days.
Results & Discussion: The microgels exhibited a 96% volume change within 37°C-43°C temperature range of interest, indicating significant potential for free volume generation upon thermal triggering. The nanocomposite hydrogels were confirmed to be mechanically strong, hydrolytically degradable, superparamagnetic, and minimally cytotoxic. We confirmed our proposed mechanism of release by showing that both the microgels and SPIONs need to be present to facilitate enhance released from this system. The microgels in thesecomposites allow for optimized control over release when heated from physiological temperatures (37°C) rather than room temperature (22°C), physiological temperature (37°C), and a hyperthermic temperature (43°C) (Fig. 2).
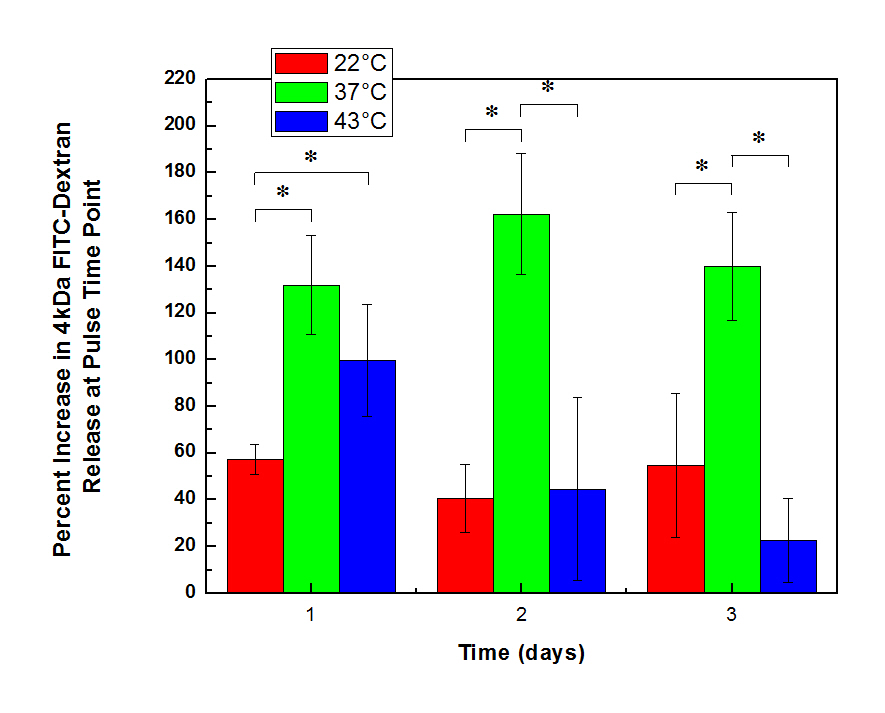
Figure 2: Percentage increases in FITC-dextran release incubated at different baseline temperatures[3].
Increasing the microgel volume fraction, optimizing the phase transition temperature of the microgel to achieve the maximum possible volume change upon relevant triggering temperature changes, and minimizing the swelling response of the bulk gel all enhance the magnitude and/or duration of pulsatile release that can be achieved, with up to 4-fold enhancements in release between the on/off state of pulsatile release achievable.
Conclusion: Combining thermosensitive microgels with magnetic nanoparticles within an in situ-gellable hydrogel scaffold offers significant advantages in terms of facilitating triggered changes in drug release using a minimally-invasive device delivery (injection) and non-invasive triggering technology (AMF).
J.P. Bickell Foundation (Medical Research Grants).; Natural Sciences and Engineering Research Council of Canada Discovery Grant.; Natural Sciences and Engineering Research Council of Canada Vanier Scholarship program.
References:
[1] Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vázquez, E. ACS Nano 2015, 9(5), 4686-4697.
[2] Campbell, S. B.; Patenaude, M.; Hoare, T. Biomacromolecules 2013, 14, 644–653.
[3] Campbell, S.; Maitland, D.; Hoare, T. ACS Macro Lett. 2015, 4, 312–316.