Introduction: Collagen materials have the ability to enhance wound healing and to facilitate functional repair. However, most of these collagen materials exhibit low resistance to degradation and mechanical stability. Therefore, exogenous cross-linking methods are widely used for collagen stabilisation. Unfortunately, chemical cross-linking is associated with impaired healing and foreign body reaction[1]. Specifically, macrophage interactions with cross-linked collagen materials have recently been demonstrated to play an important role in tissue remodelling outcomes; however, little is known about the underlying biological mechanism involved[2]. Within this context, our work explores modified material properties by cross-linking and to look for alternative cross-linking methods, based on plant extracts and polyethylene glycol (PEG). It is hypothesised that collagen films can be optimally cross-linked with PEG and plant extracts to induce adequate stability, while modulating macrophage response.
Materials and Methods: Collagen type I films were cross-linked with 0.625% glutaraldehyde, 50 mM carbodiimide, 1 mM 4S-StarPEG, 0.625% genipin or 0.1% oleuropein. Stability was assessed by mechanical testing, DSC, swelling and collagenase assay. In vitro inflammatory response was assessed using human monocyte-derived THP-1 macrophages and subsequently by morphology, alamarBlue™, PicoGreen and multiplex assay for the main inflammatory cytokines. Statistical significance was set at p<0.05.
Results: 4S-StarPEG and genipin were the only methods that preserved the fibrillar structure of collagen.
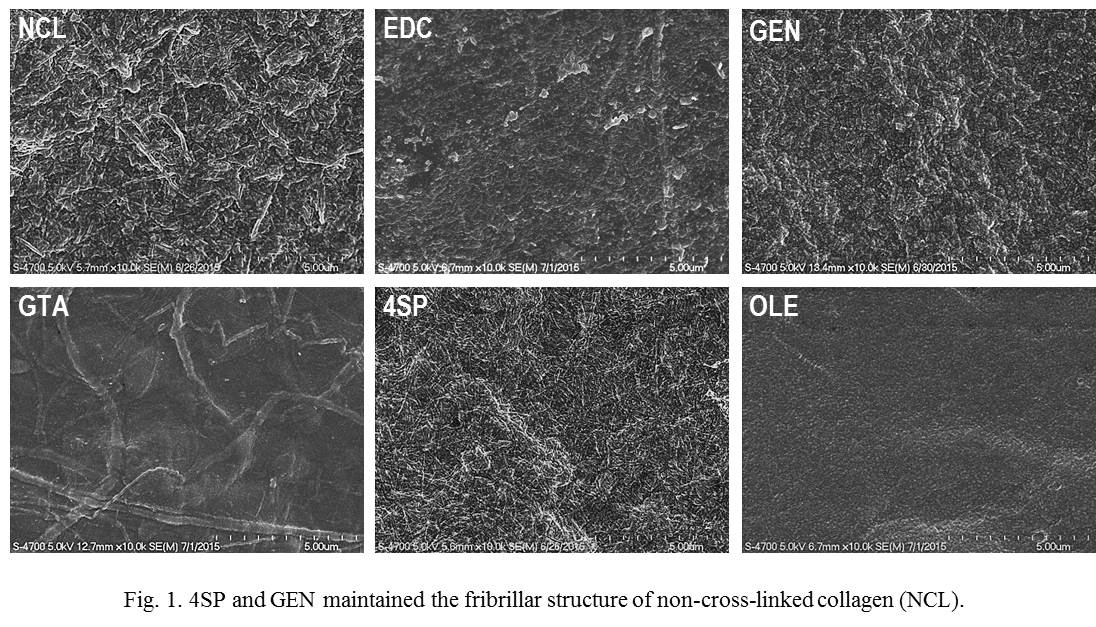
Direct addition of carbodiimide and oleuropein into collagen solutions decreased free amine groups of collagen, whilst they did not increase enzymatic stability. Only 4S-StarPEG and genipin provided an enzymatic stability equivalent to glutaraldehyde cross-linked collagen films without increasing toxicity, while inducing a similar release profile of pro-inflammatory cytokines to non-cross-linked films.
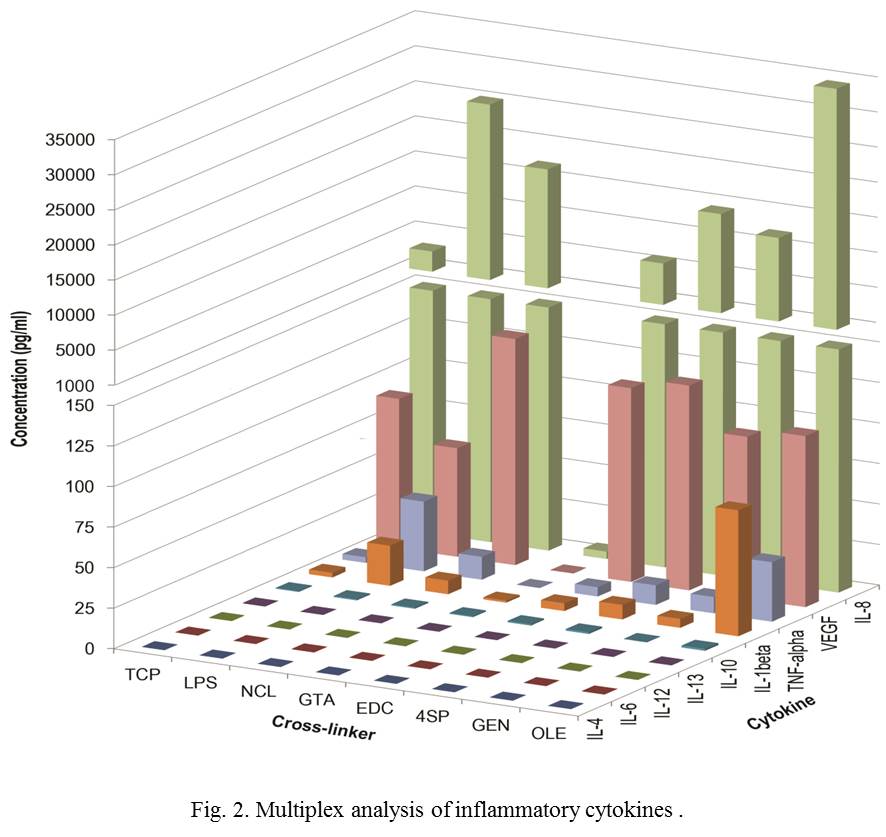
Moreover, cell culture assays using pre-conditioned media strongly suggest that sub-products potentially released from cross-linked collagen films are not responsible for the macrophage alteration. This is most likely associated with modifications in the collagen surface and structure by chemical cross-linking.
Conclusions: Overall, this study advocates 4S-StarPEG and genipin as alternative cross-linkers for tissue engineering scaffolds. Moreover, we believe that macrophage alterations is most likely associated with surface and structure modifications because we did not observe effects from potential sub-products and the only two conditions that preserve fibrillar structure presented similar release profile of pro-inflammatory cytokines to non-cross-linked films.
EU-7FP-263289-GNM
References:
[1] Delgado L. Tissue Eng Part B. 3, 298, 2015.
[2] Brown B. Acta Biomater. 9,4948, 2013.