Introduction: The targeting of lymph nodes (LN), tissues where organized lymphocyte accumulation in the body occurs, has the potential to improve the treatment of a variety of pathologies, such as B and T cell malignancies as well as sentinel LN metastasis. To achieve localized delivery to LN, principles of nanoparticle (NP)-mediated lymphatic targeting are often employed, with previously demonstrated in vivo success in tumor immunotherapy models[1]. However, within the LN, solutes and particulates are filtered based on size, with smaller molecules penetrating more deeply into LN follicles than large particulates by virtue of uptake within LN conduits[2]. A NP system optimized for lymphatic uptake that is engineered to subsequently release its payload once in lymph has the potential to improve the delivery of payload to target cells. To this end we have developed a two-stage system that combines 30 nm thiolated poly(propylene sulfide) NP (SH-NP) with thiol-reactive oxanorbornadiene (OND) linkage chemistry that degrades in a pH- and polarity-insensitive manner at programmable half-lives ranging from hours to days to release small-molecule cargo (Figure 1).
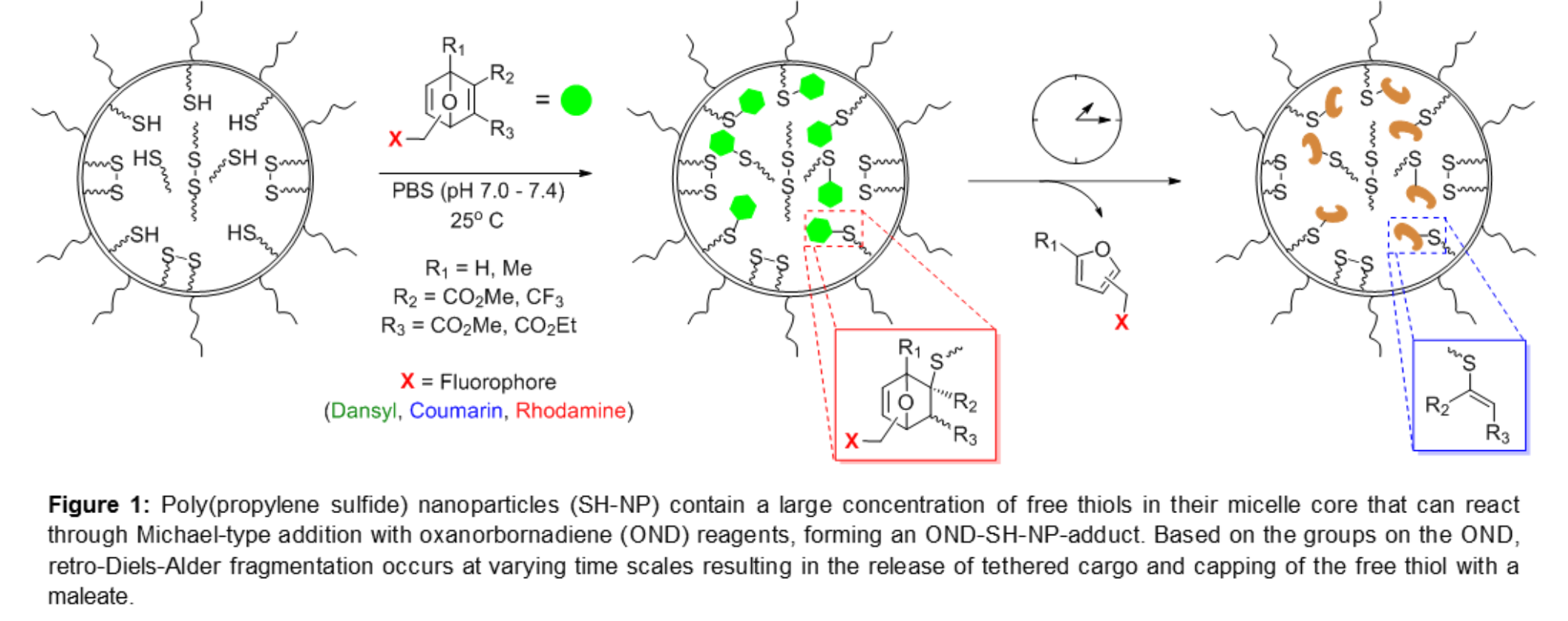
Materials and Methods: SH-NP were synthesized as previously reported[3]. SH-NP free thiols were reacted with five different OND electrophiles[4] and degradation times of the OND-SH-NP adducts were determined via dialysis at 37oC using a 20 kDa MWCO. Preliminary in vivo experiments were performed to determine the extent to which OND formulation affected LN accumulation. SH-NP were then conjugated to one of four OND: 1, 1’, 3, and 4, and then injected intradermally in the forearms of C57BL/6J mice. After 24h both axillary and brachial LN were excised and homogenized and their fluorescence measured. Collagen hydrogels at 7.5 mg/mL at physiological pH were also prepared as reported elsewhere[5]. Labeled SH-NP were conjugated to two OND variants each with different dyes and this solution was added to the collagen gel. Fluorescent images were taken over the course of 12 h at 37oC to measure the penetration of OND cargo relative to SH-NP.
Results and Discussion: The release rates from SH-NP of OND variants exhibited a wide range of half-lives from 20 minutes up to permanent linkage (Figure 2A). Decreasing OND half-life was found to dramatically decrease LN accumulation in vivo (Figure 2B). OND-mediated release from NP also increased payload penetration into collagen hydrogels in vitro (Figure 2C).
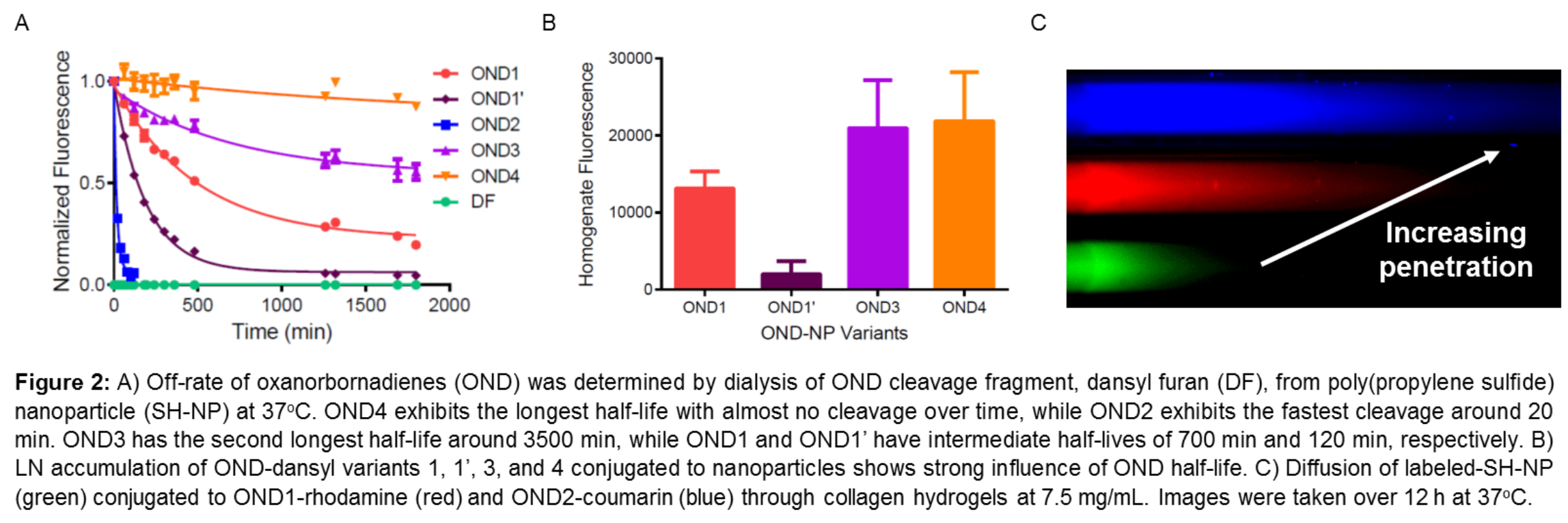
Conclusion: We demonstrate that cargo release from SH-NP can be precisely tuned to exhibit dramatically different half-lives which affect both in vitro penetration and in vivo LN accumulation. These results suggest that LN accumulation needs to be balanced against cargo penetration for delivered payload to effectively reach target cells. The benefits of this multistage drug delivery system will be explored for their potential to improve LN-targeted therapy via the enhanced delivery of payload to LN-resident cells inaccessible to existing particulate drug carrier systems currently used for LN drug delivery applications.
References:
[1] Peer, D. et al. Nat. Nanotechnol. 2007
[2] Roozendaal, R. et al. Int. Immunol. 2008
[3] Schudel, A. et al. Adv Healthc Mater. 2015
[4] Kislukhin, A.A. et al. J.A.C.S. 2012
[5] Wong, C. et al. Proc Natl Acad Sci U S A. 2011