Introduction: As the average age of the world population increases and people continue physical activity later into life, the demand for long-lasting joint replacements also rises. To assure performance and durability, implants first must be tested in conditions that closely replicate the in vivo environment. It has been suggested that excess bacteria present in the lubrication of in vitro studies may have an effect on polypeptide degradation, therefore affecting wear rates in vitro[1]. Sodium azide (SA) may be used as an antibacterial in wear simulation testing, however, previous research suggests that SA may be ineffective in preventing microbial growth[2]. It was hypothesized that SA would not effectively inhibit of bacterial growth at recommended concentrations, and increasing SA concentration could affect wear rates of ultra-high molecular weight polyethylene (UHMWPE) in a hip wear simulation.
Materials and Methods: Wear testing on crosslinked (5Mrad) and remelted UHMPWE hip inserts was conducted on an orbital bearing hip simulator (Shore Western, Monrovia, CA) following ISO14242-2:2000(E) and ISO14242-3:2009(E). Bovine serum (Hyclone, Logan, UT) was diluted to 18 mg/mL total protein and supplemented with 20mM EDTA (calcium stabilizer) and sodium azide at concentrations of 0.056, 0.2 or 5.0% (w/v). Six wear test intervals were performed with 500,000 cycles per interval for a total of 3.0 million cycles (MCyc). The UHMWPE acetabular inserts (n=3 for each test group, ID=36mm, OD=56mm) were articulated against 36mm diameter CoCrMo metal femoral heads. Wear rates were determined gravimetrically using linear regression of the cumulative wear. A two-tailed student’s t-test with equal variance as well as a one-way ANOVA with Fisher’s multiple comparison test were used to determine significant differences (α=0.05).
Serum samples from each station were plated thrice during each interval (initial, after 48 hours of testing, and at the conclusion) on agar (Trypticase Soy Agar, Becton Dickinson and Co., Sparks, MD). Plates were incubated for 24-72 hours to evaluate bacterial growth. Initial and final serum samples from each interval and each test station were evaluated for total protein concentration (Pierce™ BCA Protein Assay Kit, Thermo Fisher Scientific, Grand Island, NY) to determine SA impact on protein degradation.

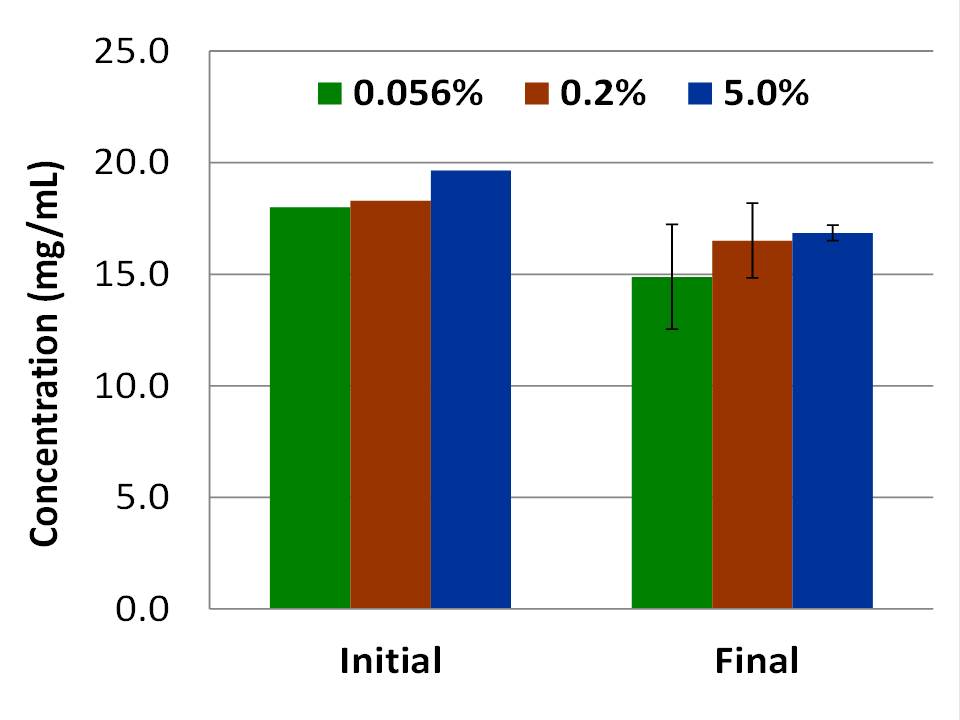
Results and Discussion: Wear rates (Fig 1) were determined to be statistically significantly different when comparing all test groups (95% confidence interval) using ANOVA and t-test analyses (p-values in Fig 1). All plates incubated with the initial serum samples did not grow bacteria, confirming the solution was free from bacteria prior to the start of each interval. After 48 hours of testing, only the samples with 5.0% SA exhibited some inhibition of bacterial growth. At the conclusion of each 0.5MCyc interval, all serum samples from testing stations, regardless of SA concentration, exhibited high bacterial growth on the agar plates. Protein concentration was observed to slightly decrease throughout the duration of the testing intervals (Fig 2).
Conclusions: Wear rates in test groups 0.056%, 0.2%, and 5% significantly decreased as the concentration of sodium azide increased. Sodium azide demonstrated minimal effectiveness at inhibiting bacterial growth withall concentrations tested.
References:
[1] J.-M. Brandt, et al. J.Eng. Medicine.2012.
[2] J. Fredericks, et al. BMES Annual Meeting, 2013.