Introduction: In-vitro irritation screening is important to ensure the safety and compliance of topical drug products. Conventional in vivo animal testing poses issues with accuracy and ethical concerns[1]. The demand for utilizing alternative methods such as three-dimensional human skin equivalents (HSEs) for screening safety and efficacy of topical formulations is increasing[2]. The purpose of this study was to (a) investigate the use of a novel material, porcine acellularized peritoneum matrix (APM)3 as a substrate to construct full-thickness HSEs, and (b) validate the use of constructed APM-HSEs as in-vitro irritation screening tool.
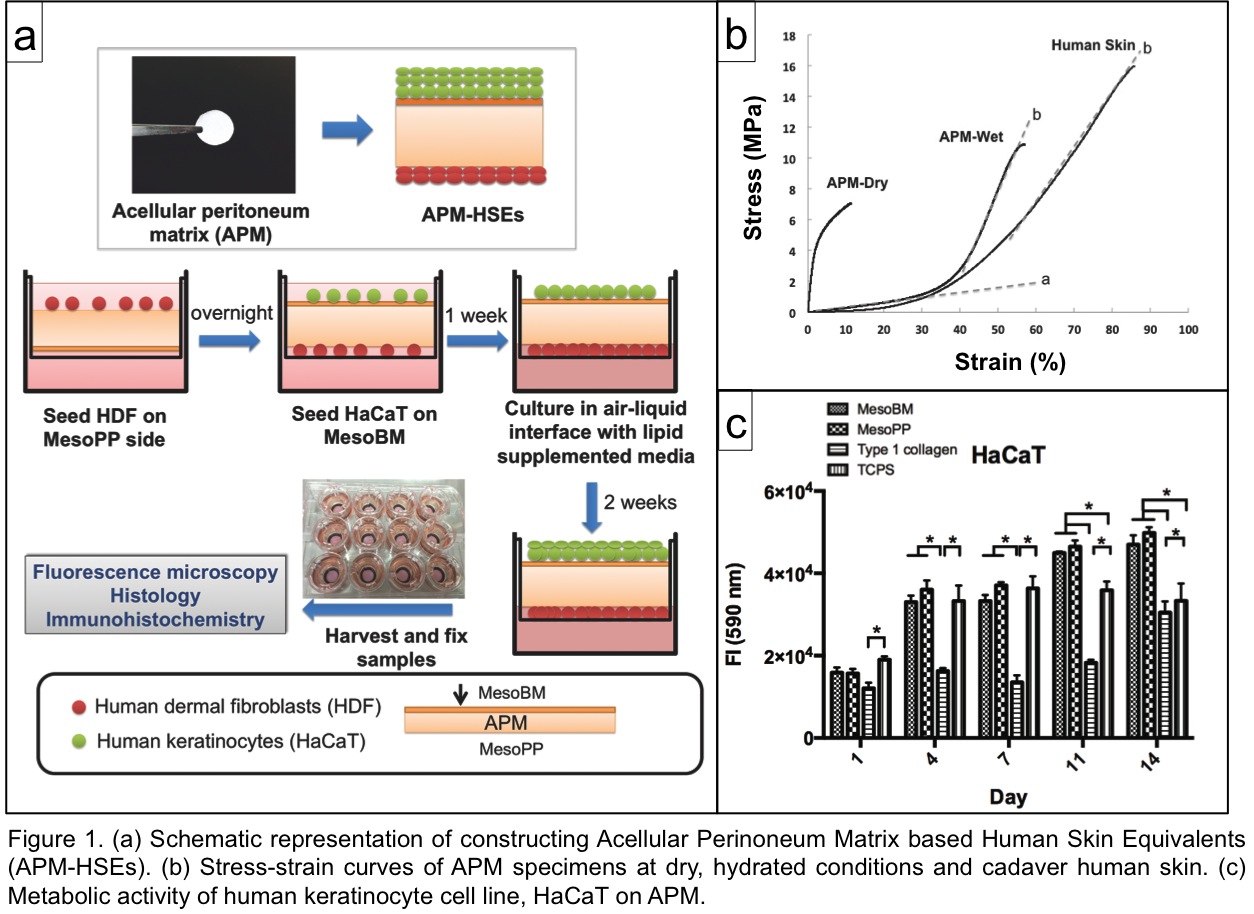
Materials and Methods: The APM was produced from porcine peritoneum using DSM Biomedical’s (Exton U.S.A.) OPTRIX™ processing method[3]. The APM-HSEs were constructed by co-seeding HDF (to the collagen side) and HaCaT[4] (to the basement membrane side) of APM disc (d= 12 mm). After submerging in culture media, the skin models were cultured at air-liquid interface for another two weeks and then harvested at week three (Fig. 1a). APM-HSEs were characterized by H&E and immunohistochemistry assays. The in-vitro irritation prediction ability of APM-HSEs was tested 42 hours post treatment. End point analysis of cellular viability (AlamarBlue® assay) and inflammatory markers were analyzed (ELISA and Bio-Plex multiplex system).
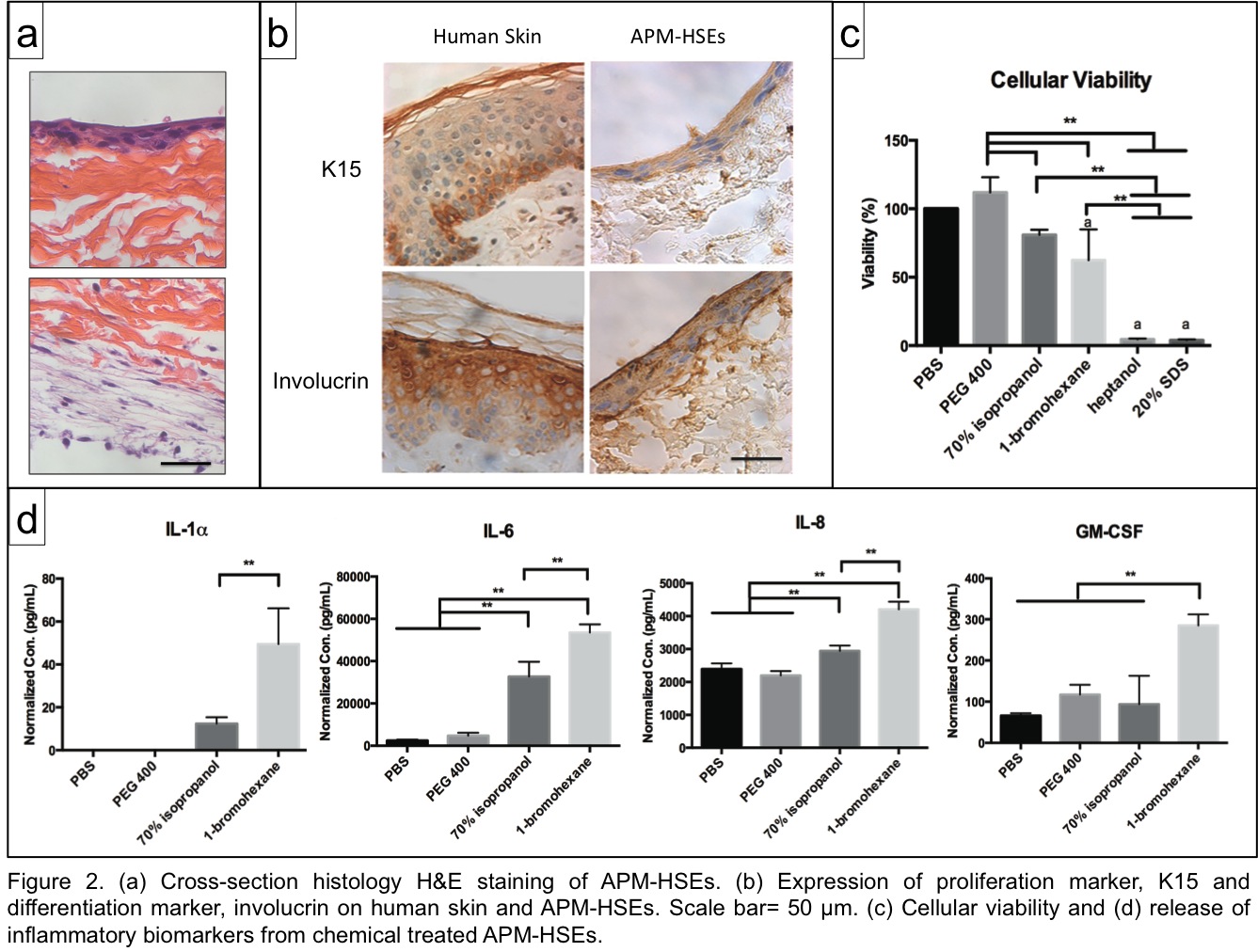
Results: APM in hydrated conditions exhibited a similar Young’s modulus as cadaver human skin (Fig. 1b). For cellular attachment, HDF and HaCaT were able to attach to both side of APM with efficiencies > 80% The metabolic activity over 14 days was significantly higher than control groups, i.e., tissue culture plate and type 1 collagen gel (Fig. 1c). The APM-HSEs possess viable, multiple cell layers (Fig. 2a) on both the basement membrane side and collagen side of the APM.
Expression of both the proliferation (Keratin 15) and differentiated (involucrin) markers was evident (Fig. 2b). For the in-vitro irritation, six proficiency chemicals (phosphate buffered saline, polyethylene glycol 400, isopropanol, 1-bromohexane, heptanol, sodium dodecyl sulfate) were applied to APM-HSEs. The viability of APM-HSEs decreased as the irritation potency of the chemicals increased (Fig. 2c). The cut-off value to separate irritants from non-irritants was 71.7% (by Receiver Operating Characteristic method). Moreover, levels of inflammatory cytokines (IL-1, IL-6, IL-8, GM-CSF) in these treatment groups further assisted the irritancy ranking (Fig. 2d).
Conclusion: Our results demonstrated the potential of APM as a substrate to construct HSEs. The porcine APM supports the attachment and growth of HDF and HaCaT. The epidermis of the APM-HSEs expressed proliferation and differentiated markers. More importantly, the constructed HSEs may have potential for in-vitro irritation screening based on their ability to accurately predict the ranking of chemicals via cellular viability and cytokine secretions.
Kathleen Roberts; Joachim Kohn; Yong Mao; Meng-Chen Lo
References:
[1] Phillips, L., 2nd, Steinberg, M., Maibach, H.I., and Akers, W.A. A comparison of rabbit and human skin response to certain irritants. Toxicol Appl Pharmacol 21, 369, 1972.
[2] Zhang, Z., and Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 4, 26, 2012.
[3] Hoganson, D.M., Owens, G.E., O'Doherty, E.M., Bowley, C.M., Goldman, S.M., Harilal, D.O., Neville, C.M., Kronengold, R.T., and Vacanti, J.P. Preserved extracellular matrix components and retained biological activity in decellularized porcine mesothelium. Biomaterials 31, 6934, 2010.
[4] Boukamp, P., Petrussevska, R.T., Breitkreutz, D., Hornung, J., Markham, A., and Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106, 761, 1988.