Introduction: Microfluidics reliably produces single and multiple emulsions with uniform size that can be applied in a wide variety of fields, such as material synthesis[1], single-cell analysis[2],[3], protein crystallization[4], enzymatic activity assay[5]-[7], and bacterial or mammalian cell culture[8],[9]. Among many other applications, drug delivery using microfluidic droplets is attractive due to their manipulability and versatility for encapsulation and release of both polar and non-polar drugs. However, the lack of chemical reactivity and selectivity of commercial surfactants, which are on the interface of droplets and involved in their stabilization, limits functionalization and manipulation of surface properties of the droplet-based drug delivery system. In the present study, we present an efficient method to surface-functionalize microfluidic droplets by modifying hydroxyl—the terminal group of the surfactant Pluronic F-127—into amino, carboxyl, N-hydroxysuccinimide ester (NHS), maleimide and biotin. We propose that these functional derivatives of Pluronic F-127 can act as surfactants for microfluidic droplets and achieve surface modifications via electrostatic adsorption, covalent conjugation and site-specific avidin-biotin interaction, respectively.
Materials and Methods: The amino (NH2), carboxyl (COOH), N-hydroxysuccinimide ester (NHS), maleimide (MAL) and biotin-terminated Pluronic F-127s were prepared through chemical modification of the hydroxyl groups of F-127. Monodisperse oil-in-water (O/W) or water-in-oil-in-water (W/O/W) microdroplets were generated by using polydimethylsiloxane (PDMS) microfluidic chips in the presence of one or multiple functional surfactants and fluorinated oil HFE-7500. FITC-labeled heparin, Cy3-labeled poly (ethylenimine) (PEI), amino-containing doxorubicin, thiol-containing 5-((2-(and 3)-S-(acetylmercapto) succinoyl)amino) fluorescein and Texas Red-labeled streptavidin were used as model molecules for surface modification of F-127-NH2, F-127-COOH, F-127-NHS, F-127-MAL and F-127-Biotin-stabilized droplets, respectively.
Results and Discussion: The above five derivatives of F-127 can act as surfactants to stabilize the droplet interface and prevent coalescence of both single and double emulsions, demonstrating that terminal modification of F-127 will not affect its inherent properties as a surfactant. Droplets with positively or negatively charged surface were produced when amino or carboxyl-terminated F-127 was used as the surfactant during the generation process in the microfluidic chip, respectively. Subsequently, dye-labeled anionic heparin and cationic PEI could coat the droplets by electrostatic interaction as evidenced by fluorescence signals on the surface of the droplets (Fig. 1A). The NHS and maleimide-modified droplets can anchor amino and thiol-containing model fluorescence dye on the surface via amidation and thiol-maleimide “click” reaction, respectively (Fig. 1B). In another scenario of modification, streptavidin could be attached to the surface of droplets stabilized with biotin-functionalized surfactant. The above three types of interaction/reaction are highly efficient and orthogonal, offering potential for multiple modifications simultaneously. To the best of our knowledge, this is the first example reported on surface modification of microfluidic droplets by using end-group-functionalized surfactants.
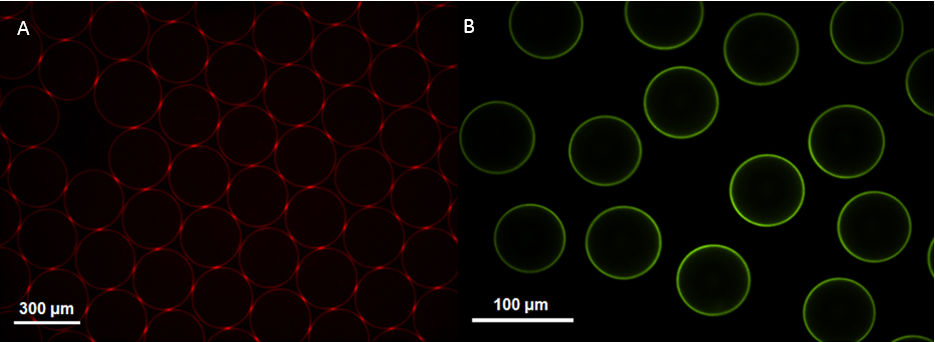
Conclusion: We have developed a versatile and efficient platform for surface modification of microfluidic droplets based on functional F-127 surfactants. The present work also suggests that surface modification is a promising technique for fabricating microfluidic droplet-based multifunctional drug delivery systems with tunable surface properties.
Support to this research by NIH and AO Foundation is acknowledged
References:
[1] Wang W, Zhang M-J, Chu L-Y. Functional Polymeric Microparticles Engineered from Controllable Microfluidic Emulsions. Accounts of Chemical Research. 2014;47:373-84.
[2] Wang BL, Ghaderi A, Zhou H, Agresti J, Weitz DA, Fink GR, Stephanopoulos G. Microfluidic high-throughput culturing of single cells for selection based on extracellular metabolite production or consumption. Nat Biotechnol. 2014;32:473-8.
[3] Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell. 2015;161:1187-201.
[4] Zhu Y, Zhu LN, Guo R, Cui HJ, Ye S, Fang Q. Nanoliter-scale protein crystallization and screening with a microfluidic droplet robot. Sci Rep. 2014;4:5046.
[5] Chiu Y-L, Chan HF, Phua KKL, Zhang Y, Juul S, Knudsen BR, Ho YP, Leong KW. Synthesis of Fluorosurfactants for Emulsion-Based Biological Applications. ACS Nano. 2014;8:3913-20.
[6] Juul S, Ho YP, Koch J, Andersen FF, Stougaard M, Leong KW, Knudsen BR. Detection of single enzymatic events in rare or single cells using microfluidics. ACS Nano. 2011;5:8305-10.
[7] Juul S, Nielsen CJF, Labouriau R, Roy A, Tesauro C, Jensen PW, Harmsen C, Kristoffersen EL, Chiu YL, Frøhlich R, Fiorani P, Cox-Singh J, Tordrup D, Koch J, Bienvenu AL, Desideri A, Picot S, Petersen E, Leong KW, Ho YP, Stougaard M, Knudsen BR. Droplet microfluidics platform for highly sensitive and quantitative detection of malaria-causing plasmodium parasites based on enzyme activity measurement. ACS Nano. 2012;6:10676-83.
[8] Zhang Y, Ho YP, Chiu YL, Chan HF, Chlebina B, Schuhmann T, You L, Leong KW. A programmable microenvironment for cellular studies via microfluidics-generated double emulsions. Biomaterials. 2013;34:4564-72.
[9] Chan HF, Zhang Y, Ho YP, Chiu YL, Jung Y, Leong KW. Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment. Sci Rep. 2013;3:3462.