Statement of Purpose: Hydrogels with tunable stiffness are highly useful in studying cellular mechano-transduction in 3D. A recent work by Anseth et al. has demonstrated this concept[1]. The stiffness of this gel was tuned by a light-mediated cis-trans conformational change of azobenenze. However, the changes in gel stiffness were low (about 160 Pa) and potentially limited their biological applications. Supramolecular chemistry has been used widely for forming physically assembled hydrogels. For instance, macromers functionalized with cyclodextrin (CD) and with azobenzene or adamantane (AD) are used to form ‘host-guest’ interactions, which result in macroscopic physical gelation[2]. We hypothesize that supramolecular assembly can be used to tune the mechanical properties of chemically crosslinked hydrogels. Here, we present a cytocompatible thiol-allylether hydrogel with reversibly tunable stiffness by means of CD-AD host-guest interactions.
Methods: A step-growth thiol-allylether hydrogel was formed by thiolated poly(vinyl alcohol) (TPVA), 4-arm PEG-allylether (PEG4AE) and mono-allylether functionalized CD (CD-AE) in the presence of photoinitiator LAP and light (365 nm, 10 mW/cm2). To tune the stiffness of hydrogel, hydrogel was incubated in either (1) 5 wt% of PEGAD solution for 36 hours at room temperature and transferred back to pH 7.4 PBS, or (2) alternative PEGAD (5 wt%) and CD (1 wt%) incubation for 36 hours at room temperature (Figure 1A). In situ photo-rheometry and strain-sweep oscillatory rheometry were performed on a Bohlin CVO 100 digital rheometer to determine the mechanical properties of the hydrogels. Pancreatic β-cells (MIN6, 2x106 cells/mL) or pancreatic cancer cells (COLO-357, 5x106 cells/mL) were encapsulated within thiol-allylether hydrogels to evaluate the cytocompatibility of this system. Cell viability was evaluated by live/dead staining and confocal imaging.
Results: The gelation of TPVA-PEGAE-CD thiol-allylether hydrogels was evaluated by in situ photorheometry (Fig. 1B). The gelation was highly efficient, as demonstrated by the rapid gel point of merely ~3 seconds. The final gel modulus was about 1,300 Pa. To stiffen the hydrogels, a TPVA-PEGAE-CD gel was incubated in 5 wt% of PEGAD solution for 36 hr (Fig. 1C). We found that the gel stiffness increased almost three-fold to ~3,000Pa. The level of gel stiffening is sufficient to induce difference in cellular signaling (data not shown). Since soluble PEGAD interacted with immobilized CD in the hydrogel via supramolecular interactions, the gradual dissociation of PEGAD from immobilized CD caused a decrease of modulus from ~3,000 Pa to ~1,700 Pa in about a week. Furthermore, these hydrogels can be stiffened and softened alternatively. In addition to the stiffening caused by PEGAD, a more rapid gel softening (within hours) could be achieved by adding unmodified CD in PBS (Figure 1D, from ~3000 Pa to ~2000 Pa). More importantly, the process of reversing hydrogel stiffness was repeatable by alternating incubation condition between PEGAD and CD (Figure 1D). Note that control hydrogels in Figure 1C and 1D were completely incubated in PBS through the length of study. Finally, live/dead staining results showed that this hydrogel system is highly cytocompatible to MIN6 and COLO365 cells (Figure 1E, 1F).
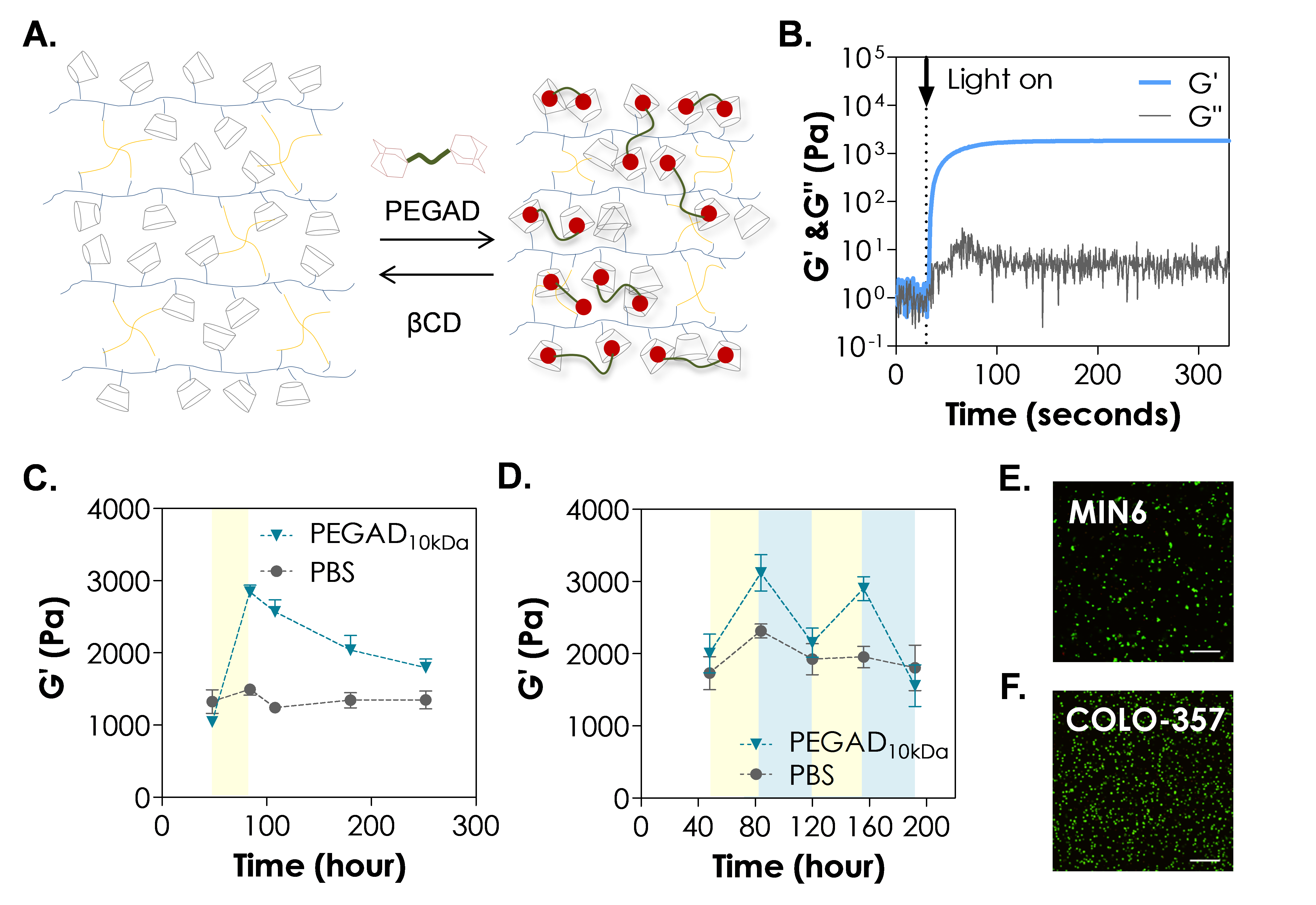
Figure 1. (A) Schematics of the reversible stiffening and softening of thiol-allylether hydrogel using PEGAD and CD, respectively. (B) In situ photorheometry of thiol-allylether hydrogel containing CDs. (C) Effect of PEGAD on stiffening of thiol-allylether hydrogel. (D) Effect of PEGAD and CD on reversibly stiffening and softening of thiol-allylether hydrogels. Yellow and blue region indicate PEGAD and CD exposure, respectively. Confocal images of (E) MIN6 and (F) COLO357 cells-laden hydrogel stained with Live/Dead staining kit on day 1 (scales = 200 μm). Gel formulations: 1.5 wt% TPVA, 1.5 wt% PEG4AE, and 22 mM of CD-AE.
Conclusions: In summary, we have demonstrated that hydrogel stiffness could be reversibly tuned by host-guest interactions between network conjugated CD and soluble PEGAD. This hydrogel system was cytocompatible to in situ encapsulation of MIN6 and COLO357 and should be of great interest in the study of biomechanical properties on cell fate processes. Current work is focusing on evaluating the effect of matrix stiffness on the gene expression of MIN6 and pancreatic cancer cells.
This work was supported in part by the NIH (R21CA188911) and NSF (CAREER #1452390).
References:
[1] Rosales AM et al. Biomacromolecules. 2015(16)798-806.
[2] Rodell CB et al. Biomacromolecules 2013(14)4125-34.