Osseous tissue forms in various physiological circumstances, ranging from normal long bone development, to abnormal extraskeletal bone formation and callus-mediated bone repair. These contexts, normal or otherwise, share many cellular and biomechanical characteristics, which are poorly understood due to the inability to isolate individual factors in early-phase bone formation.
Developing new methods for producing ossified tissue is essential for both understanding the fundamental processes underlying early bone development as well as for tissue engineering and tissue grafting following trauma or injury.
In this context, the current work focused on developing a tissue-engineered 3D cell model of fracture callus repair.
Periosteum cells, the main determinants in the reparative phase of bone healing, were extracted from murine sources and seeded in a pilot study into fibrin clot scaffolds, analogous structurally and biochemically to the early-phase callus formation microenvironment.
To simulate the biomechanics of the fracture repair process, the cell-hydrogel system was manipulated by inserting two bone-like calcium phosphate anchors on the base of the culture dishes; around which the cells organized the fibrin gel over time, replacing it with matrix and giving rise to an aligned, mineralized collagen structure. Thus, cells in the 3D constructs underwent a wide spectrum of physiologically-relevant stages of bone repair, including blood clot remodelling, matrix production and mineralization.
Similarly to fracture repair, in this 3D system, mineralization starts from the bone-like ends and progresses throughout the construct over several days (Figure 1).
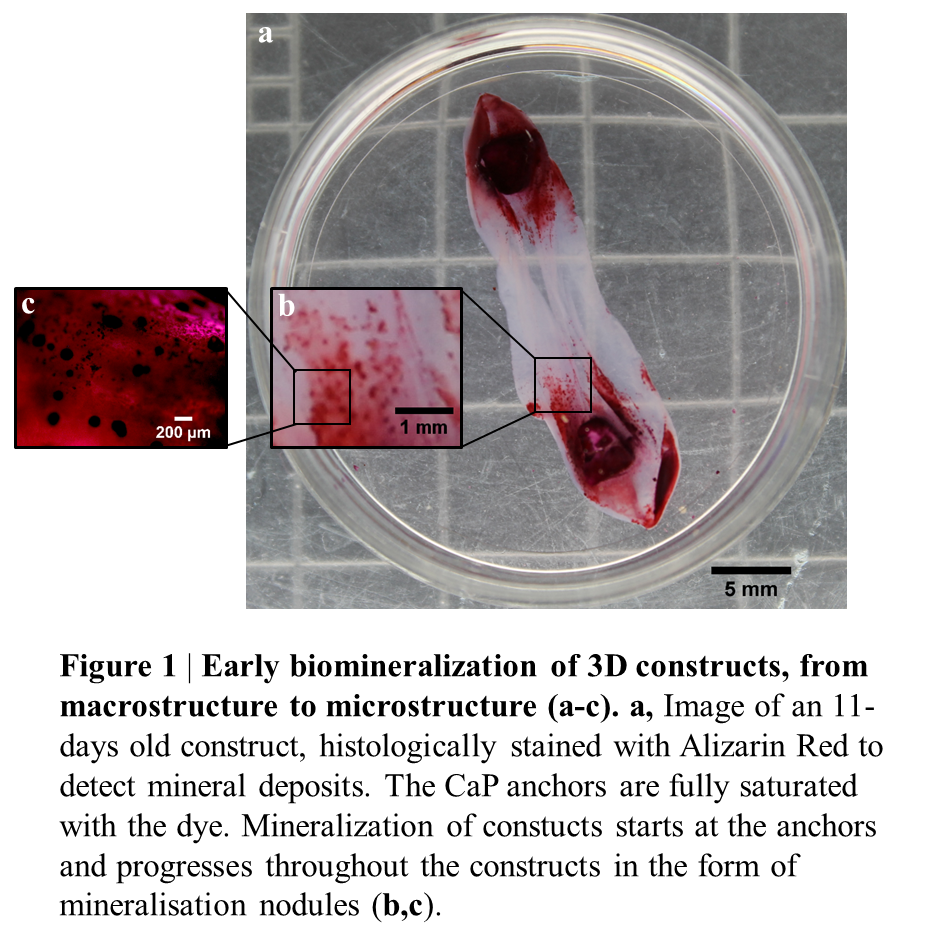
Confocal Raman spectroscopy confirmed that the mineral formed within the matrix is OCP, an intermediate of Hydroxyapatite and differs from the anchor material.
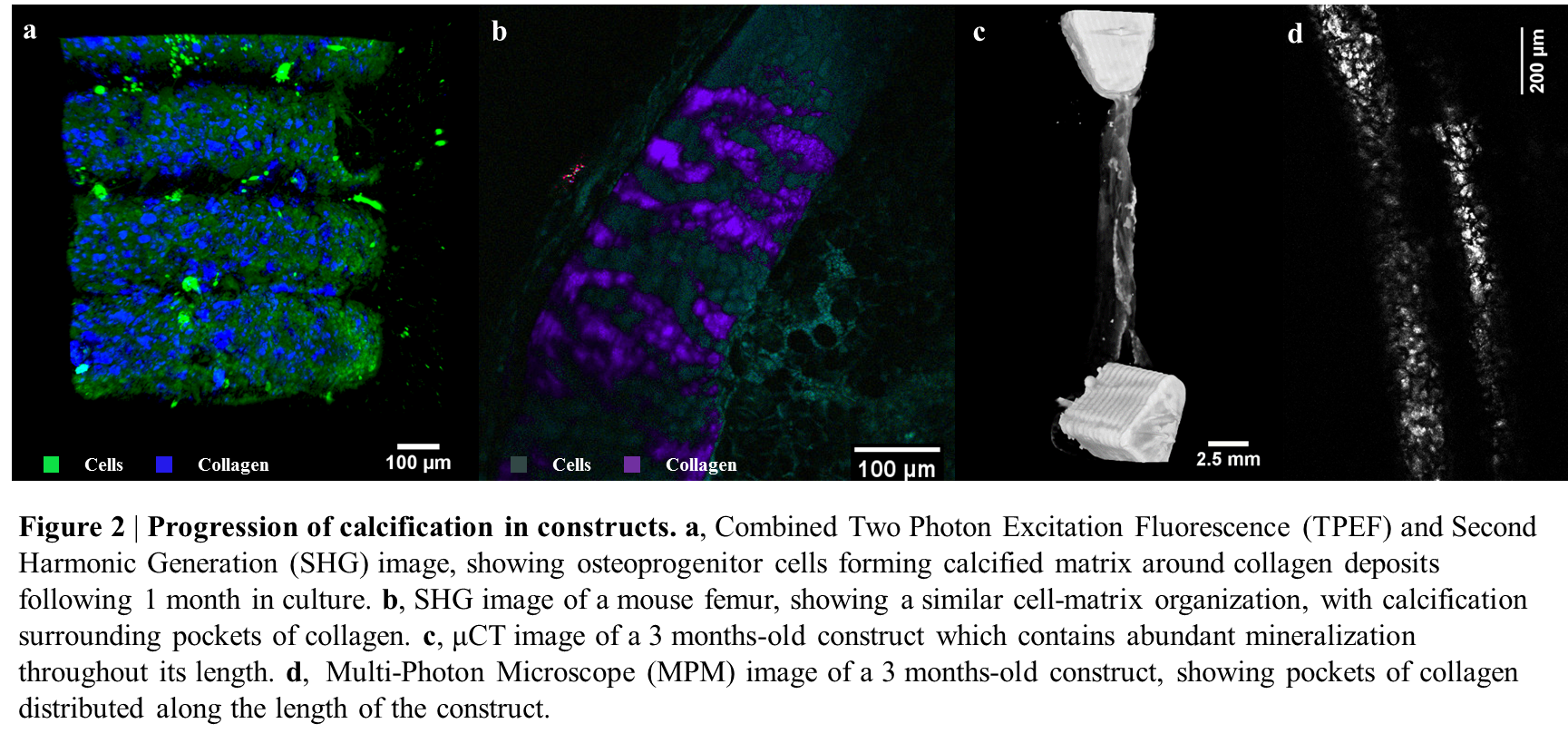
Following 1 month in constructs, these cells showed a high degree of calcification around collagen deposits compared to controls, as revealed by Two-Photon Excitation Fluorescence and Multi-Photon Microscopy with SHG (Figure 2a). This calcification pattern was found to be extremely similar to the one in murine femurs (Figure 2b).
Full osteogenic medium supplementation of periosteum cell constructs over longer time periods (3 months) gave rise to a considerably mineralized structure (Figure 2c) and organized collagen deposition (Figure 2d).
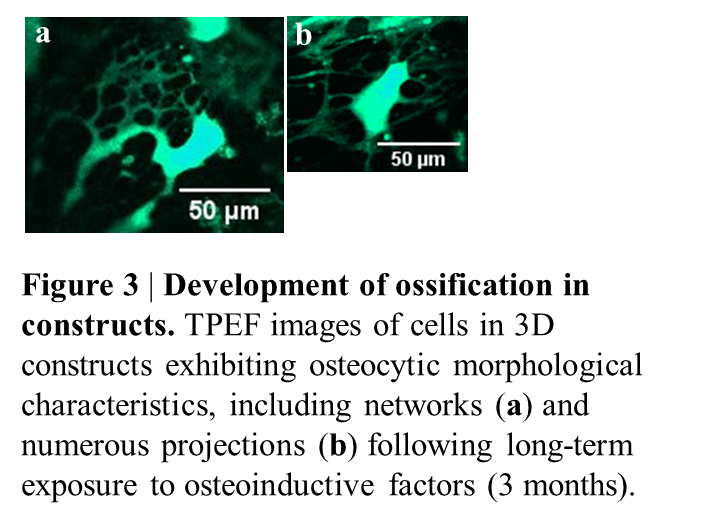
Following 3-6 months and treatment with osteogenic factors, the cells started exhibiting osteocyte-like features, including cell networks (Figure 3a) and many projections (3b) and expressed early and late osteocytic markers (podoplanin and sclerostin). Raman spectroscopy on 3 months-old samples revealed strong 958 cm−1 bands resembling carbonated apatite found in bone mineral, suggesting a cell-mediated conversion of disordered and amorphous OCP to the apatite structure of bone mineral.
This novel model displays promise for use in fracture repair and heterotopic ossification research as well as for bone tissue engineering.
Professor Sara Rankin; Dr Harsh Amin; Dr Clarence Yapp