Introduction: Congenital heart defects are the most common type of birth defect and the leading cause of infant death. Current repair strategies involve surgical operations and the use of fixed autografts and homografts, which inevitability require repeat surgeries due to their inability to grow with the patient and a mechanical mismatch with native tissue. Congenital heart defects can be detected by prenatal ultrasound as early as the first trimester. The most severe defects will require surgical intervention soon after birth. This time between diagnosis and surgery can effectively be used to engineer functioning cardiac tissue. The goal of this study is to create an autologous, implantable cardiac patch that promotes the differentiation of reprogrammed human amniotic fluid derived stem cells (AFSC).
Materials and Methods: Cell source: AFSCs were attained from amniotic fluid by routine amnioreduction. Stem cells were isolated and sorted for cKit+ and reprogrammed through mRNA transfection of Yamanaka factors.
Mesoporous Silica Vectors (MSV): MSV (1µm in diameter; 51% of porosity) were loaded with GSK-3 and Wnt inhibitors separately and encapsulated in PLGA microspheres by solid-oil-water (S/O/W) emulsion method. PLGA thickness is altered to tune release mechanics. The release of GSK-3 and Wnt inhibitors from PLGA-MSV, in vitro, was performed in PBS (37oC, under mild agitation). Samples were collected up to 2 weeks.
Fibrin/PEG hydrogel: Fibrin/PEG hydrogels are formed by dissolving fibrinogen was combined at a 1:1 ratio with a bi-functional NHS poly(ethylene glycol) (PEG). After conjugation, reprogrammed AFSC with MSV were mixed into the solution. Thrombin was combined with the cell solution at a 1:4 ratio to initial fibrinogen. Cells within hydrogels were maintained in pluripotent stem cell media for 3 days and assessed for embryonic pluripotency markers. Directed cardiac differentiation of reprogrammed AFSCs was accomplished by MSV release of small molecules inhibiting the GSK3/Wnt signaling pathways.
Results and Discussion: AFSC 3 days after seeded into fibrin/PEG hydrogels maintain viability and markers of pluripotency Oct4, TRA-1-81 (Fig 1). Preliminary release studies of GSK3 containing MSV show delayed release characteristics from different formulations of the hydrogel and vectors (Fig 2). Differentiation studies within fibrin/PEG hydrogels loaded with nanoparticles show an increase in brachyury expression day 1 after the start of differentiation suggesting mesendoderm lineage. With further inhibition of the Wnt signaling pathway, encapsulated cells express early cardiac markers Nkx2.5 and Isl-1.
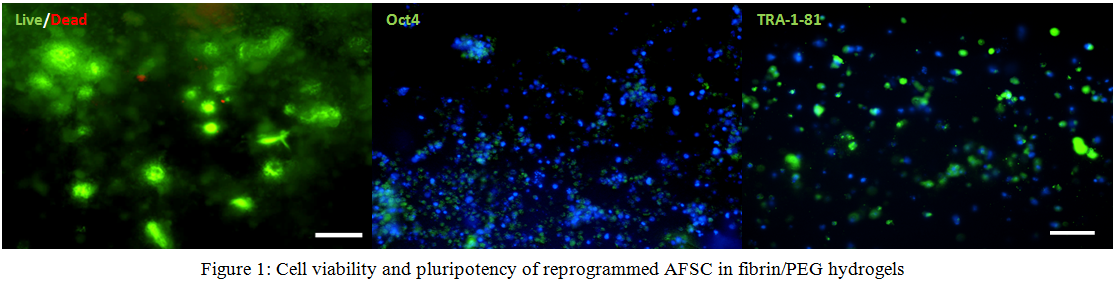

Conclusions: The current study shows potential for a completely autologous cardiac tissue patch for the repair of congenital heart defects. Successful reprogramming and differentiation of AFSCs proves that functional cardiac cells can result from amniotic fluid. Reprogrammed AFSCs in 3D show that pluripotency is maintained and directed cardiac differentiation can occur by delivery vectors releasing small molecules of the GSK3/Wnt inhibitory molecules.
Rodrigo Ruano