The synthesization of BSA-coated iron nanoparticles, and the mechanism of their uptake in the mitochondria of MCF7 breast cancer cells
-
1
McMaster University, School of Engineering Technology, Canada
-
2
McMaster University, Student-Biotechnology, School of Engineering Technology, Canada
-
3
McMaster University, Professor-Biotechnology, School of Engineering Tecnology, Canada
Objectives:
- To test the viability of MCF7 cell lysis through the magnetic properties of iron MNPs which would penetrate the breast cancer cells
- Verifying iron nanoparticle (MNP) uptake within the mitochondria of the MCF7 cells
Materials and Methods: MCF7 cells were grown for 48h then incubated with MNPs for an additional 24h to allow for uptake of the particles into the cells. Iron MNPs are synthesized by a modified Massart coprecipitation method dissolving 1.72g of Iron (II) chloride(FeCl2 4H2O) and 4.67g of Iron (III) chloride(FeCl3 6H2O). The solution was then added to 20 mL ammonium hydroxide(NH4OH) and stirred. The resulting brown precipitate was then centrifuged for 15 min at 5000g and the pellet redispersed in deionized water to remove unreacted reagents and to decrease the pH. After repeating the centrifugation process five times, the MNPs were then pelleted again and redispersed in 20 ml of 5% (w/v) aqueous solution of BSA. In these experiments a batch of MNPs were made with FITC-BSA for imaging.
Coating MNPs with BSA caused some aggregation, and sonication was performed for 1 hour using a probe sonicator to separate the MNPs into individual particles. The sonicator pulsed on and off in 5 second intervals to keep BSA from denaturing. The resulting dispersion was a homogeneous liquid when inspected at 400× magnification.The liquid was refrigerated for 24h and then ultracentrifuged at 100,000g for 1 hour. The pellet was redispersed in 40 mL deionized water to remove excess BSA. A 10uL sample of this dispersion was added into the MCF7 Petri-dish, and incubated for another 24h at 37〫 C. The next day, DMEM 1X media was discarded from the dish. The dish was rinsed with PBS, and cells were scraped from the surface, into a centrifuge tube. After 30s centrifugation, the supernatant was discarded. In the experiment with FITC-BSA coated particles, cells were stained according to the procedure for Mito-Tracker ®Red CMXRos FM[5]. In the experiment with BSA coated particles cells were stained according to the staining procedure for Prussian Blue Iron Stain kit[4] Note: H20 replaced with PBS. The same cells were also stained with Mito-Tracker.
Results and Discussion: The nanoparticles obtained after sonication were observed with the DLS instrument. The results of the observation showed that the FITC-BSA sample that was sonicated for 1h yielded particles approximately 169.57nm in size. Figure 1 shows the results between three runs where the size distribution is consistent and almost exact.
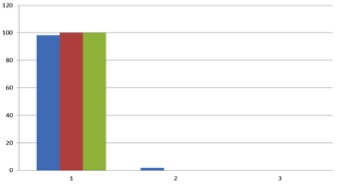
Figure 1 DLS data
MCF7 cells incubated with MNPs were observed under an epi-fluorescent microscope to observe MNP uptake within the cell. The image in Figure 2 shows the cells absorbing MNPs coated in FITC-BSA. Figure 2 also shows successful staining of the mitochondria using Mito-Tracker Red FM fluorescent dye. The fluorescent staining seen in the overlay fluorescent image confirms that the nanoparticles are within the mitochondira of the MCF7 cells.
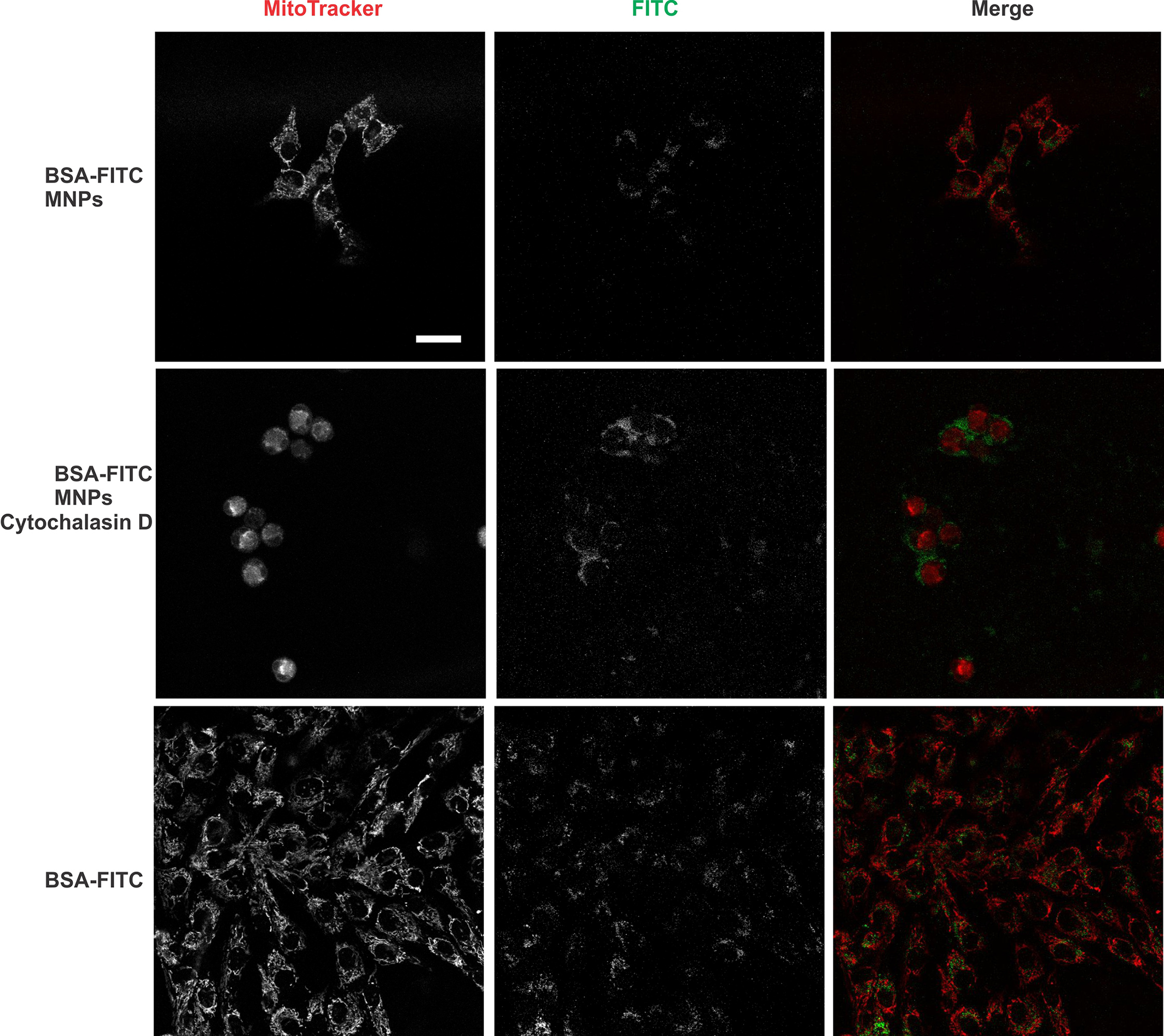
Figure 2 FITC-BSA and Mito-Tracker dye
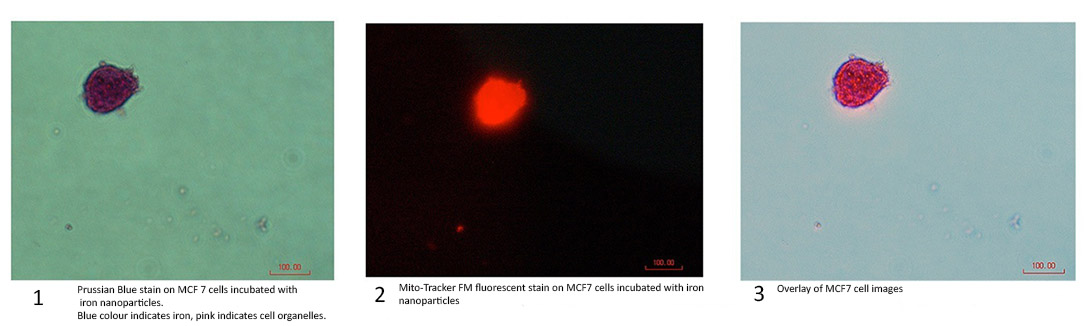
Figure 3 Prussian Blue iron stain
The results from the epi-fluorescent microscope in Figure 3 confirm the cells successfully absorbing the nanoparticles in the first photo.The cells are stained to induces the Prussian Blue reaction, typically used on tissues to detect the presence of iron.[2] Iron will stain a bright blue andorganelles will stain pink. [3] Image one in Figure 3 clearly shows this phenomenon, proving that iron MNPs have entered the cell. Image two shows the same cell, after being stained with Mito-Tracker. The third image is an overlay, thus proving that FITC-BSA is not what is attracting the iron nanoparticles to the mitochondria.
These results prove a novel method in which iron nanoparticles enter the mitochondria of MCF7 breast cancer cells.
Paula Gremmen; Dean Ishwar Puri; Dr. Suvojit Ghosh; Eric Mahoney; Dr. James F Hainfeld
References:
[1] Hainfield, J. F. (2015). Curing Cancer with Magnetic Nanopaticles . Retrieved April 7, 2015, from nanoprobes.com: http://www.nanoprobes.com/newsletters/2013-08-Curing-Cancer-with-Magnetic-Nanoparticles/curing-cancer-with-magnetic-nanoparticles-video.html
[2] Parry, Nicola. (May 7 2013). Prussian Blue - A Histology Stain. Retrieved June 5 2015 from bitesizebio.com: http://bitesizebio.com/13441/feeling-blue-try-prussian-blue/
[3] Polysciences Inc. (June 26 2006). Prussian Blue Reaction for the Demonstration of Iron. Retreived June 5 2015 from: http://www.polysciences.com/skin/frontend/default/polysciences/pdf/601.pdf
[4] Sigma Aldrich (2015) Iron Stain Kit retrieved from: http://www.sigmaaldrich.com/catalog/product/sigma/ht20?lang=en®ion=CA&gclid=CMr_mYuOncgCFYVAaQod4uEM-w
[5] Thermofisher Scientific (2015) MitoTracker® Mitochondrion-Selective Probes retrieved from: https://tools.thermofisher.com/content/sfs/manuals/mp07510.pdf
Keywords:
in vitro,
cell,
nanoparticle
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials for cancer therapy
Citation:
Adham
M,
Reid
E,
Scardamaglia
D and
Geng
F
(2016). The synthesization of BSA-coated iron nanoparticles, and the mechanism of their uptake in the mitochondria of MCF7 breast cancer cells.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02466
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.