Introduction: Screw-type titanium alloy implants are routinely used in orthopedics to stabilize fractured bone and allow natural healing. New demands now exist for screw fixation systems demonstrating the capacity to sustain loads while allowing motion. A new titanium screw type was therefore developed, offering a novel dynamic thread design, which utilizes an expandable crest component instead of a fixed one. Following insertion, the resulting thread has the ability to expand into areas of low bone density and exert a radial force with an improved initial fixation for osseointegration. The intent of this study was to evaluate the preliminary safety and efficacy of this design using a sub-chronic ovine model.
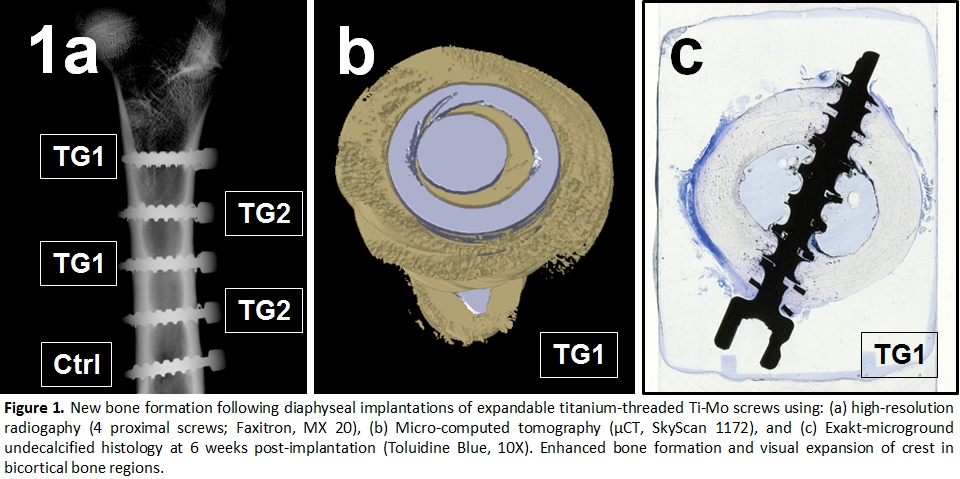
Materials and Methods: Biomaterials were composed a high-strength Ti-15Mo screw shank (Ø4.5mm; Intelligent Implant Systems) with an expandable thread crest made of either TiAlV alloy (TG1, n=4), commercially pure titanium (TG2, n=4) or crestless (Ctrl, n=2). Following an IACUC-approved protocol as well as AAALAC and CCAC regulations, a female Dorset Rideau Arcott hybrid sheep (>12mo.; 40kg) was placed in lateral recumbency to receive a total of 10 bilateral femoral diaphyseal and bicortical screw insertions leaving a 2-mm gap between the screw heads and the femoral surface. At 6 weeks, bone ingrowth was reconstructed by Micro-CT (SkyScan 1172) and imaged by high-resolution radiography (Faxitron MX-20). Explanted screws were sectioned longitudinally, processed for undecalcified histology, infiltrated with PMMA, microground (Exakt 400 CS) and stained with Toluidine Blue. The resulting slides were examined histologically and then scanned using a NanoZoomer Digital Pathology 2.0-HT for histomorphometry. Using Aperio ImageScope, new bone formation was measured in cortical, periosteal, and endosteal areas.
Results: Both TiAlV (TG1) and cpTi (TG2) expandable threaded screw types exhibited variable amounts of new bone formation (Figure 1) and prominent osteoblasts without evidence of cartilage formation. New bone formation was graded as mild to moderate at the periosteal surface and minimal to mild at the endosteal surface and cortex. The new bone adjacent to the expandable threaded implants was predominantly lamellar with a minimal woven bone component, consistent with normal bone formation and remodeling. Good osseointegration, characterized by moderate to marked bone apposition to the implant surface with minimal fibrous tissue at the bone-implant interface was seen in all groups. Histopathology results indicated an excellent safety and biocompatibility in all test and control groups as evidenced by a mild tissue reaction. Histomorphometry also revealed increased new bone formation surrounding the expandable threaded implants when compared to controls.
Discussion: High-strength Ti-15Mo screws containing either a TiAlV or cpTi expandable crest showed minimal tissue reaction with no local adverse effects and excellent biocompatibility. Histomorphometry demonstrated increased new bone formation surrounding the expandable threaded implants, but a larger study is necessary to accurately quantify this difference.
Conclusion: With good osseointegration and bone remodeling, this dynamic technology has the potential to be applied to various threaded components in the spinal and orthopedics fields and may represent a new approach to implant fixation.