Introduction: Silicon nitride (SiN) is an advanced ceramic that has shown potential as an orthopaedic biomaterial in its bulk form and as a coating material on cobalt-chromium (CoCr) substrates. Its high mechanical strength, low friction characteristics, low wear rates and particle dissolution characteristics are suited for next generation longer lasting total hip replacements[1],[2]. Moreover, SiN has been shown to have osseointegration properties[3],[4]. However, there is limited evidence to support its biocompatibility as an implant material[5],[6]. The aim of this study was to investigate cytotoxicity, inflammatory cytokine release and genotoxicity (DNA damage) of peripheral blood mononuclear cells (PBMNCs) isolated from healthy human volunteers to SiN nanoparticles and CoCr wear debris.
Materials and Methods: SiN nanopowder (<50 nm, Sigma UK) and CoCr wear particles (nanoscale, generated in a multidirectional pin-on-plate reciprocator using methodologies previously developed at University of Leeds[7]) were heat-treated for 4 h at 180°C and dispersed by sonication for 10 min prior to their use in cell culture experiments. Whole peripheral blood was collected from healthy donors (ethics approval BIOSCI 10-108, University of Leeds). The PBMNCs were isolated using Lymphoprep® as a density gradient medium and incubated for 24 h in 5% (v/v) CO2 at 37∘C to allow attachment of mononuclear phagocytes. SiN and CoCr particles were then added to the phagocytes at a volume concentration of 50 µm3 particles per cell and cultured for 24 h in RPMI 1640 culture medium in 5% (v/v) CO2 at 37∘C. Cells alone were used as a negative control and lipopolysaccharide (LPS; 100 ng/ml) was used as a positive control. Cell viability was measured after 24 h by ATPLite assay (Perkin Elmer) and tumour necrosis factor alpha (TNF-α) release was measured by sandwich ELISA (Diaclone). Results from cell viability assays and TNF-α response were expressed as mean ±95% confidence limits and the data was analysed using one-way analysis of variance (ANOVA) and Tukey-Kramer post-hoc analysis. Single and double-stranded DNA damage in the cells was measured by using alkaline comet assay (Tevigen). Hydrogen peroxide (100 µM) was used as a positive control and cells alone as a negative control.
Results and Discussion: At a high volume concentration of particles (50µm3 per cell), SiN did not affect the viability of PBMNCs, while CoCr significantly reduced the viability over a 24 hour period [Figure 1A]. Additionally, CoCr particles caused significantly elevated levels of pro-inflammatory cytokine TNF-α, whereas no inflammation was associated with SiN particles [Figure 1B]. The Comet assay detected no DNA damage in cells cultured with SiN particles, whereas CoCr wear debris caused noticeable damage to the DNA [Figure 2].
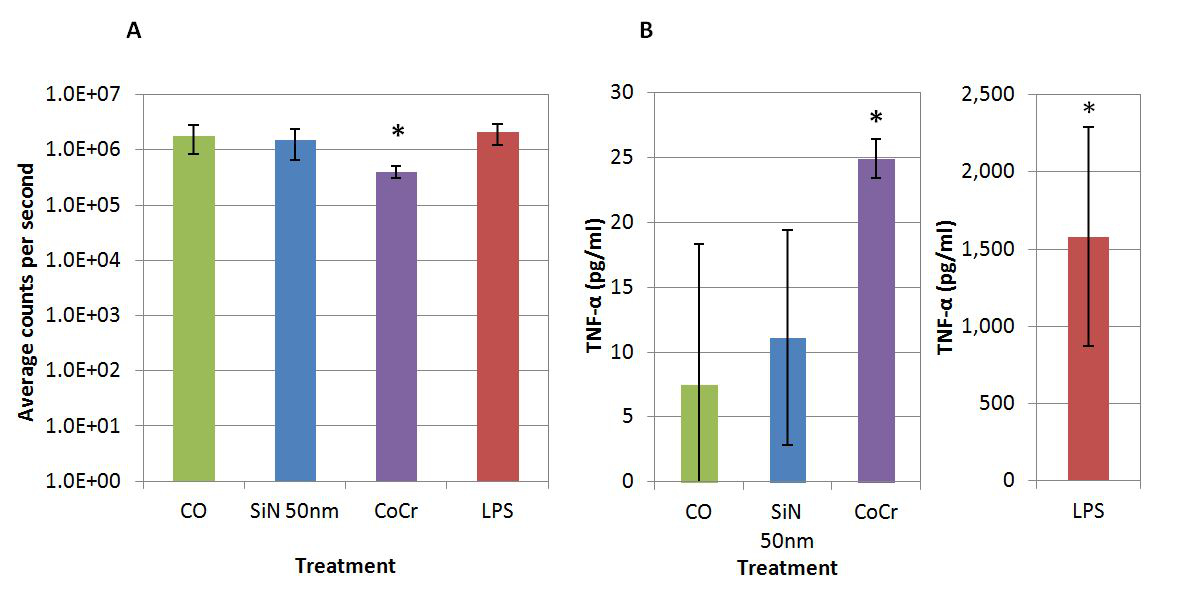
Figure 1. A) Viability of peripheral blood mononuclear cells (PBMNCs) co-cultured with silicon nitride (SiN) 50nm model particles and cobalt chromium (CoCr) wear debris at 50 µm3 particles per cell. B) TNF-α release in PBMNCs co-cultured with SiN 50nm model particles and CoCr wear debris at 50 µm3 particles per cell. CO: Cells only control, LPS: Lipopolysaccharide positive control. *Significant difference from the cell only control (ANOVA and Tukey-Kramer post hoc test, p<0.05).
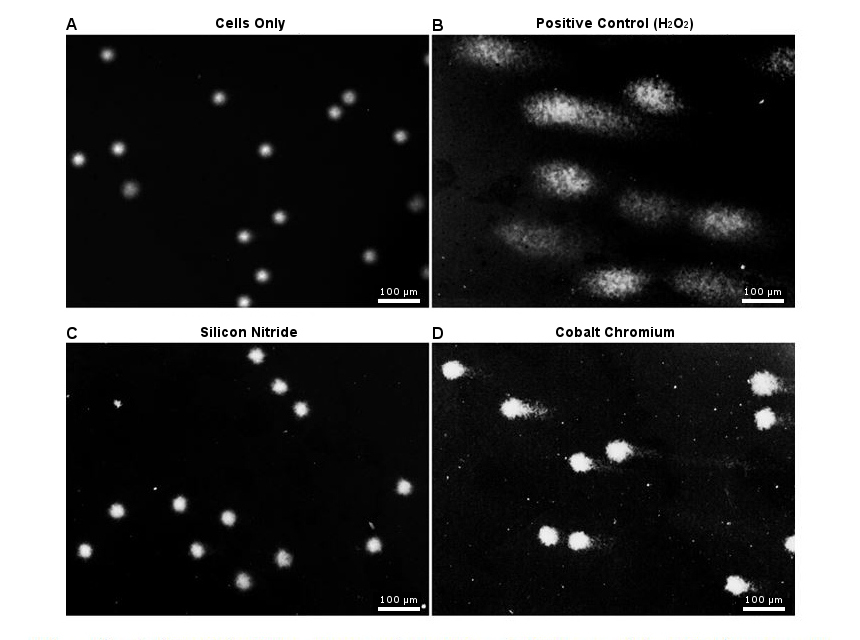
Figure 2. Single-cell gel electrophoresis images of PBMNCs. A) No noticeable DNA damage seen in cells only control. B) Extensive DNA damage seen in positive control (100 µM hydrogen peroxide). C) No noticeable DNA damage seen in cells co-cultured with silicon nitride 50nm model particles. D) Visible DNA damage seen in cells co-cultured with cobalt chromium particles.
Conclusion: This study has demonstrated the in-vitro biocompatibility of SiN nanoparticles with primary human monocytic cells. Therefore, SiN is a promising orthopaedic bearing material not only due to its suitable mechanical and tribological properties, but also due to its biocompatibility.
The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. GA-310477 LifeLongJoints.
References:
[1] Bal, B. S. et al. Fabrication and testing of silicon nitride bearings in total hip arthroplasty: winner of the 2007 “HAP” PAUL Award. J. Arthroplasty 24, 110–6 (2009).
[2] Olofsson, J., Grehk, T. M., Berlind, T., Persson, C. & Jacobson, S. Evaluation of silicon nitride as a wear resistant and resorbable alternative for THR. 2, 94–102 (2012).
[3] Guedes e Silva, C. C. et al. Bone growth around silicon nitride implants—An evaluation by scanning electron microscopy. Mater. Charact. 59, 1339–1341 (2008).
[4] Webster, T. J., Patel, A. A., Rahaman, M. N. & Sonny Bal, B. Anti-infective and osteointegration properties of silicon nitride, poly(ether ether ketone), and titanium implants. Acta Biomater. 8, 4447–54 (2012).
[5] Zhang, Y. F., Zheng, Y. F. & Qin, L. A comprehensive biological evaluation of ceramic nanoparticles as wear debris. Nanomedicine 7, 975–82 (2011).
[6] Neumann, A., Jahnke, K., Maier, H. R. & Ragoss, C. Biocompatibilty of silicon nitride ceramic in vitro. A comparative fluorescence-microscopic and scanning electron-microscopic study. Laryngorhinootologie. 83, 845–51 (2004).
[7] Germain, M. a. et al. Comparison of the cytotoxicity of clinically relevant cobalt–chromium and alumina ceramic wear particles in vitro. Biomaterials 24, 469–479 (2003).