Targeting urothelial tumors of upper urinary tract with drug-eluting stents impregnated by supercritical fluids
Alexandre
Barros1, 2, 3,
Shane
A.
Browne3, 4,
Carlos
Oliveira2, 5,
Rui
L.
Reis1, 2,
Estevão
E.
Lima2, 5,
Ana
R.
Duarte1, 2 and
Kevin
E.
Healy3, 6
-
1
3B´s Research Group - Biomaterials, Biodegradables and Biomimetics, University of Minho, Headquarters of the European Institute of Excellence on Tissue Engineering and, Portugal
-
2
ICVS/3B's - PT Government Associate Laboratory, University of Minho, Portugal
-
3
University of California, Berkeley, Department of Bioengineering, United States
-
4
National University of Ireland Galway, Centre for Research in Medical Devices (CÚRAM), Ireland
-
5
Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Portugal
-
6
University of California, Berkeley, Department of Materials Science and Engineering, United States
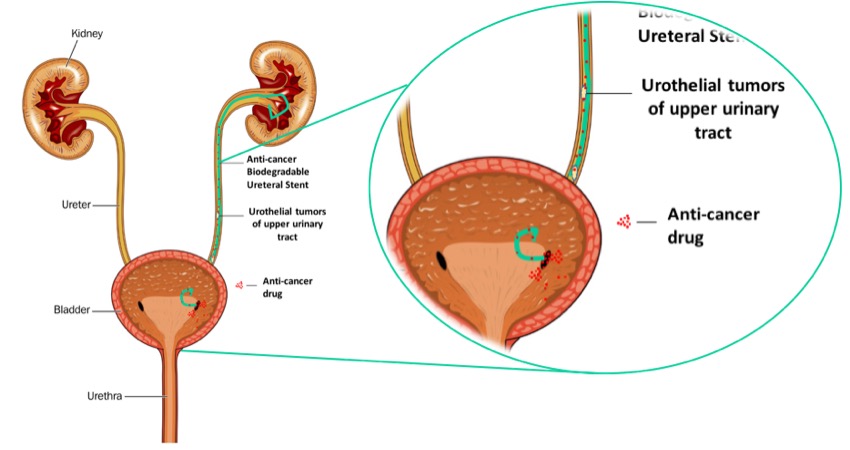
Urothelial tumors of upper urinary tract are ranked among the most common types of cancers worldwide and it has been considered as one of the more expensive to treat due of its long-term propensity of recurrence. The current standard therapy to prevent recurrence is intravesical Bacillus Calmette–Guerin (BCG) immunotherapy, but it presents several disadvantages such as BCG failure and intolerance. Another way is to use chemotherapy, that has been reported to be generally better tolerated that BCG. In this case, drugs such as epirubicin, doxorubicin, paclitaxel and gemcitabine are used. Nevertheless, intravesical chemotherapy only prevents recurrence in the short-term[1],[2]. These failings can be partially attributed to the short residence time and low penetration of the drug within the upper urinary tract and the cancer cells, resulting in a need for frequent drug instillation[3]. To avoid these problems, biodegradable ureteral stents impregnated by supercritical fluid CO2 (SCF) with each of the four anti-cancer drugs were produced (figure 1). Four types of drug-eluting biodegradable stents were studied, impregnated with paclitaxel, epirubicin, doxorubicin and gemcitabine. The release kinetics of the impregnated drugs from the anti-cancer drug-eluting stents was measured in artificial urine solution (AUS) for 9 days. The in vitro drugs release from the impregnated biodegradable ureteral stents was analyzed using a microplate reader. The in vitro release study in AUS showed a higher release in the first 72h for the four anti-cancer drugs impregnated after this time the plateau was achieved and the stent degrades after 9 days. Regarding the amount of impregnated drugs by SCF the gemcitabine showed higher amount (109 µg) and the lower amount was obtained for paclitaxel (67 ng). The diffusion coefficient and the impregnation yield were calculated. The anti-tumoral effect of the developed stents in transitional cell carcinoma (TCC) - T24 cell lines was evaluated. T24 cell line was exposed to graded concentrations (0.01 to 2000 ng/ml) of the four drugs for both 4 and 72 hours to determine the sensitivities to each drug (IC50). Toxicity as a result of both direct and indirect contact of the cell lines with the different material conditions of biodegradable stent were studied. The four anti-cancer drugs showed a concentration-dependent inhibitory effect on the T24 and HUVEC cell lines with IC50’s for paclitaxel of 7.30ng and 501.50ng, respectively. The T24 cell line shows to be more sensitive than HUVEC cell line for all the anti-cancer drugs tested. The direct and indirect contact of the anti-cancer biodegradable stents with the T24 and HUVEC cell lines confirm the anti-tumor effect of the stents impregnated with the four anti-cancer drugs, reducing around 75% of the viability of the T24 cell line after 72h and no killing effect in the HUVEC cells. Finally, this study has shown the killing efficacy of the anti-cancer drug eluting biodegradable stents in vitro for the T24 cell lines, with no toxicity observed in the control, non-cancerous cells.
Luso-American Foundation's Grant for Internships in the University of California, Berkeley, 2015/CON5/CAN8; FCT PhD Grant (SFRH/BD/97203/2013); European Union's Seventh Framework Programme (FP7/2007-2013) under grant agreement n° REGPOT-CT2012-316331-POLARIS; Project “Novel smart and biomimetic materials for innovative regenerative medicine approaches (Ref.: RL1 - ABMR - NORTE-01-0124-FEDER-000016)” cofinanced by North Portugal Regional Operational Programme (ON.2 – O Novo Norte), under the National Strategic Reference Framework (NSRF), through the European Regional Development Fund (ERDF)
References:
[1] Lange et al, Ureteral stent-associated complications : where we are and where we are going, Nat Rev Urol 2015, (12) 17-25
[2] Lu et al, Mucoadhesive polyacrylamide nanogel as a potential hydrophobic drug carrier for intravesical bladder cancer therapy, European Journal of pharmaceutical sciences, 2015, (72) 57-68
[3] Remzi et al, Upper urinary tract urothelial carcinoma: what have we learned in the last 4 years?, Ther Adv Urol 2011, (3) 69-80
Keywords:
Hydrogel,
Drug delivery,
Biodegradable material,
Polymeric material
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
General Session Oral
Topic:
Drug-eluting devices
Citation:
Barros
A,
Browne
SA,
Oliveira
C,
Reis
RL,
Lima
EE,
Duarte
AR and
Healy
KE
(2016). Targeting urothelial tumors of upper urinary tract with drug-eluting stents impregnated by supercritical fluids.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02657
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.