Introduction: Current chemotherapeutic regimens have shown poor success rates in many types of cancer due to severe off-target toxicity and drug resistance. Drug resistance can be intrinsic or result from up-regulation of efflux pumps, anti-apoptotic pathways or other adaptive pathways after initial therapy.[1][2] Strategies to combat drug resistance include dosing with chemotherapeutic cocktails and delivering sensitizing agents or gene therapies with chemotherapeutics. Recently, the co-delivery of small interfering RNA (siRNA) with chemotherapeutics has received considerable attention due to the ability to specifically silence target genes known to mediate drug resistance through the RNA interference pathway. However, in order to successfully deliver therapeutics intracellularly, delivery vehicles that can load and protect multiple therapeutic agents are needed. Here, the properties of cationic nanogels were modulated through strategic manipulation of crosslinking density and cationic monomers to enhance their viability as intracellular co-delivery vehicles.
Materials and Methods: Activators regenerated by electron transfer (ARGET) atom transfer radical polymerization (ATRP) was utilized to synthesize cationic nanogels via emulsion polymerization as reported by Forbes.[3] Here the effect of the cationic monomers 2-(diethylamino)ethyl methacrylate (DEAEMA) and 2-(diisopropylamino)ethyl methacrylate (DPAEMA) and non-degradable and degradable crosslinking agents tetraethylene glycol dimethacrylate (TEGDMA) and bis(2-methacryloyl)oxyethyl disulfide (SSXL) on nanogel properties were investigated.[4] Hydrodynamic size as a function of pH was measured using dynamic light scattering and dose dependent cytocompatibility of nanogels was assessed in HEPG2, human hepatocellular carcinoma cells for 24 hours.
Results and Discussion: Nanogel pKa was shifted from pH 7.15 to 6.26 as the hydrophobicity of the cationic monomer was increased (Fig 1). This trend is a result of increased polymer-polymer interactions leading to a need for increased ionization to achieve the swelling transition.[5] This result demonstrates the ability to modulate pKa so that swelling is minimized at physiological pH and maximized at the pH of early endosomes (~6-6.5). Fig 1 also shows that nanogel volume swelling decreased as crosslinking density was increased from 2.5 to 5 mole percent and decreased as crosslinker length decreased. This result demonstrates that volume swelling can be easily tuned for optimization of therapeutic loading and that nanogels can be rendered biodegradable for enhanced biocompatibility. Dose dependent cytocompatibility assays demonstrated minimal toxicity to HEPG2 cells after 24 hours at concentrations up to 1.0mg/mL and 0.10mg/mL via membrane integrity and cell proliferation assays respectively (Fig 2). This result shows that the nanogels have limited toxicity in the expected dosing range.
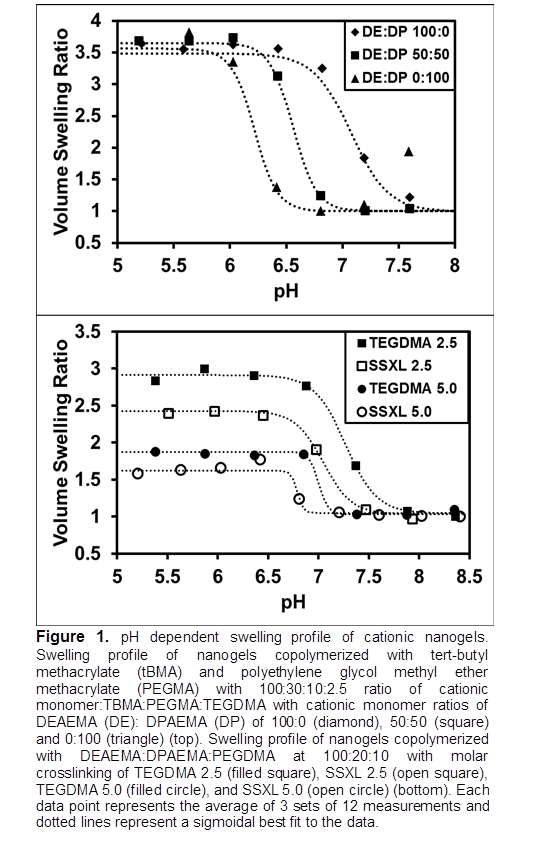
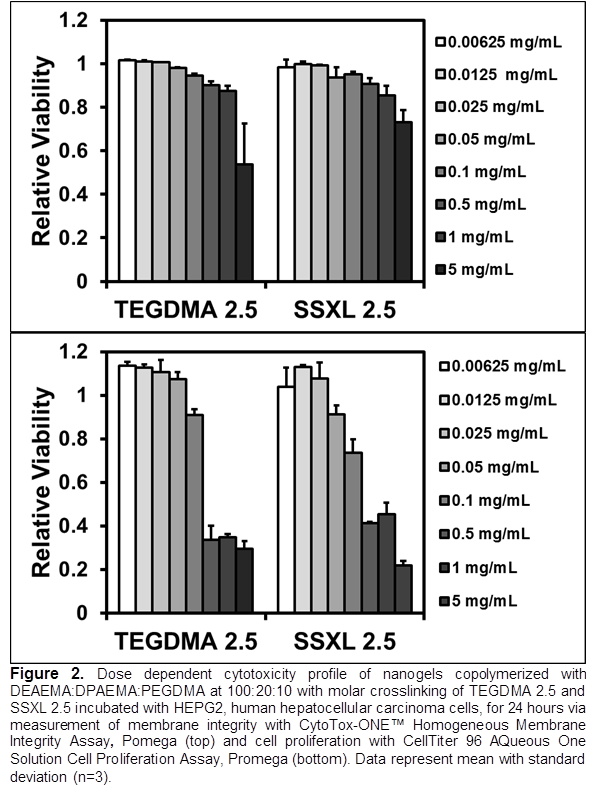
Conclusion: The properties of cationic nanogels were modulated via cationic monomer hydrophobicity and crosslinking agents. Nanogels with pH responsive transitions in the ideal range for endosomal delivery were reported. The presence of cationic charges and biodegradable chemical crosslinks will enable these materials to electrostatically bind siRNA and stably load chemotherapeutic agents making them excellent candidates for co-delivery applications.
References:
[1] Holohan, C.; Van Schaeybroeck, S.; Longley, D. B.; Johnston, P. G., Cancer drug resistance: an evolving paradigm. Nature Reviews Cancer 2013, 13 (10), 714-726.
[2] Creixell, M.; Peppas, N. A., Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today 2012, 7 (4), 367-379.
[3] Forbes, D. C.; Creixell, M.; Frizzell, H.; Peppas, N. A., Polycationic nanoparticles synthesized using ARGET ATRP for drug delivery. European Journal of Pharmaceutics and Biopharmaceutics 2013, 84 (3), 472-478.
[4] Peppas, N. A., Liechty, W.B, DELIVERY OF SMALL INTERFERING RNA AND MICRO RNA THROUGH MEMBRANE-DISRUPTIVE, RESPONSIVE NANOSCALLE HYDROGELS. 2015. http://www.freepatentsonline.com/y2015/0216814.html
[5] Siegel, R. A.; Firestone, B. A., PH-DEPENDENT EQUILIBRIUM SWELLING PROPERTIES OF HYDROPHOBIC POLY-ELECTROLYTE COPOLYMER GELS. Macromolecules 1988, 21 (11), 3254-3259.