Introduction: The decellularization of tissues has evolved as a natural extension of using allogeneic and xenogeneic grafts to replace parts of the body. Some processes have even been utilized to produce commercial products intended to repair soft tissues, such as damage to cutaneous tissue from Diabetic and Venous ulcers[1],[2]. Today, decellularization is being extended to organs to create regenerative scaffolds for organ replacement. The performance of decellularized tissues, however, has not been consistent or satisfactory. This is due to the large variation in cell component removal and disruption of the remaining extracellular matrix by decellularization processes developed to date[3]-[5]. Our decellularization research has focused on solving this problem by balancing the removal of cellular components (source of immunological rejection) while at the same time leaving the remaining extracellular matrix as unperturbed as possible to promote tissue regeneration when implanted or repopulated with cells. We have developed a novel decellularization process that provides high cell component removal balanced with minimal alteration of the remaining extracellular matrix. Here we compare the performance of our process performed on human skin to that of a similar leading commercial product.
Methods: Split thickness human skin was obtained from the Halifax Regional Tissue Bank, Halifax, NS, Canada. The skin was decellularized as described in our patent pending process (USPTO 13/580,367). Samples of a leading commercial human decellularized dermal product were obtained directly from the manufacturer. Samples from both sources were analyzed for DNA content using a PICOGreen® DNA assay, Glycosaminoglycan (GAG) content using a Blyscan® quantitation kit, and immunohistochemical analysis for known immunogenic cell determinants HLA-DR and HLA-A,B,C. In-vivo performance of decellularized dermal matrices was assessed using a sub-cutaneous, immune-competent rat biocompatibility model (ASTM standard F1408-97). In addition to the decellularized dermal samples, untreated intact human skin and a sham operation site provided +ve and –ve controls, respectively. Eight rats (n=8) were sacrificed at 1, 3, and 9 weeks post-surgery and samples with underlying muscle were retrieved for analyses. Analyses included H&E stain, immunohistochemical staining for endothelial cells (anti-CD31), T-lymphocytes (anti-CD3), T-helper cells (anti-CD4), macrophages (anti-CD68) as well staining for rat type III collagen to monitor new tissue ingrowth. Positively stained cell types were enumerated and a Student T-test was used to compare the leading commercial samples to our lab-processed samples.
Results and Discussion: Our lab-based decellularization process provided greater levels of DNA removal, higher residual GAG levels, and a complete absence of HLA-DR and HLA-A,B,C. The leading commercial product had significant levels of residual DNA, lower GAG content, and very significant staining for both HLA types. In-vivo, these differences resulted in very little evidence of remodeling of the commercial human dermal product even after 9 weeks (Fig.1). Further, analyses of cell types present at each time point revealed significantly higher levels of macrophages and T-helper cells at 9 weeks in the commercial product indicating the presence of a chronic inflammatory process (Figs. 2 & 3).
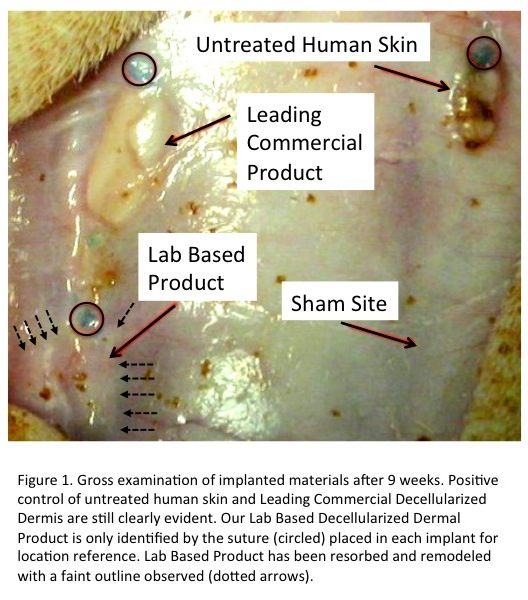
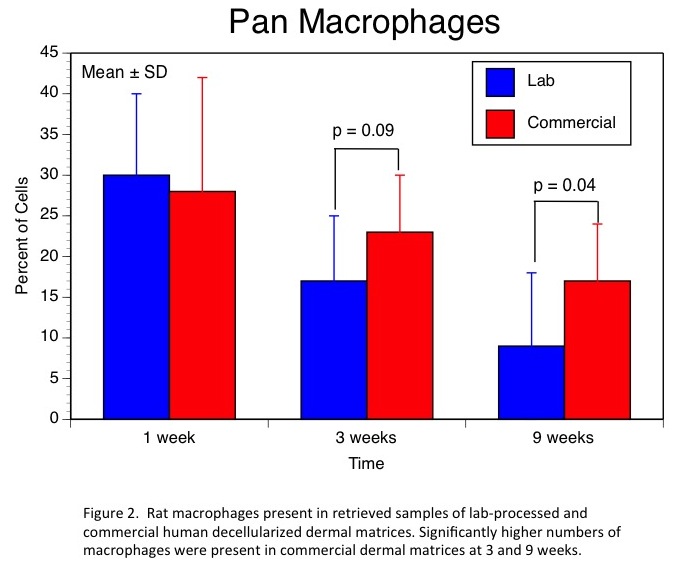
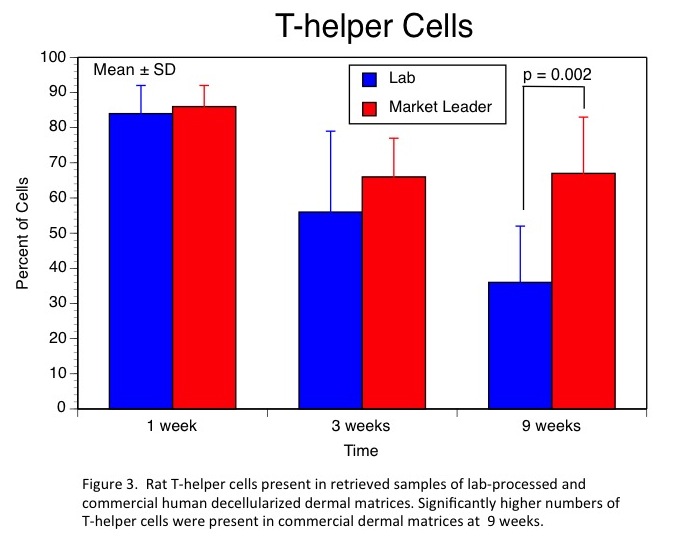
Conclusions: Our patent-pending decellularization process provided superior cell removal, maintenance of extracellular structure, and in-vivo performance of human dermal matrices. These promising results have led to human clinical trials currently underway.
This work was sponsored by a grant from the Canadian Institutes for Health Research (CIHR); The authors would like to acknowledge the support of the Halifax Regional Tissue Bank (HRTB)
References:
[1] Meezan, E., Hjelle, J. T., and Brendel, K., A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues, Life Sci 17 (1975) 1721-1732.
[2] C.R. Dyck and P.F. Gratzer, Decellularized Tissues in Tissue Engineering, in New Research on Biomaterials, Nova Science Publishers Inc., Hauppauge, NY, USA (2007), Chapter 11, pp. 281-320
[3] C. K. Mueller, S. Schultze-Mosgau, Histomorphometric analysis of the phenotypical differentiation of recruited macrophages following subcutaneous implantation of an allogenous acellular dermal matrix, Int. J. Oral Maxillofac. Surg. (2011) 40: 401–407
[4] T.W. Gilbert, J.M. Freund and S.F. Badylak, Quantification of DNA in Biologic Scaffold Materials, J. Surg. Res. (2009) 152: 135–139
[5] W.Q. Sun, H. Xu, M. Sandor and J. Lombardi, Process-induced extracellular matrix alterations affect the mechanisms of soft tissue repair and regeneration, J. Tissue Eng. (2013) 4: 1-13