Introduction: Numerous bone grafting materials have been used clinically as on osteoconductive matrix for bone repair and regeneration. Often, the bone defect has an irregular shape and size that is not easily estimated by the clinician and can result in an unsatisfactory surgery. In order to improve the handling properties of the bone grafting material, we have developed a self-expandable mineral-collagen composite (MCC) scaffold implant to guide and support bone regeneration in bone defects. The self-expansion property of the MCC has the advantage of supporting bone growth in bone defects that have irregular geometry or difficult size matching over the current materials available. In addition, the collagen matrix of the composite implant functions as a space expander that can provide ample inter-mineral particle space for cellular infiltration and bone regeneration.
Materials and Methods: In Vitro Characterization: The MCC is composed of bovine Type I collagen and bovine anorganic bone mineral. The composite implant was prepared by dispersing mineral particles in collagen fiber dispersion such that the final weight ratio of collagen to mineral was 20:80. The mixture was loaded into molds, freeze dried, crosslinked, hydrated and compressed to a predetermined size, freeze dried, packaged and sterilized. SEMs were conducted at test laboratories following standard sample preparation and method. The volume expansion of the implant was evaluated as the percentage of volume increase when compared to the initial volume.
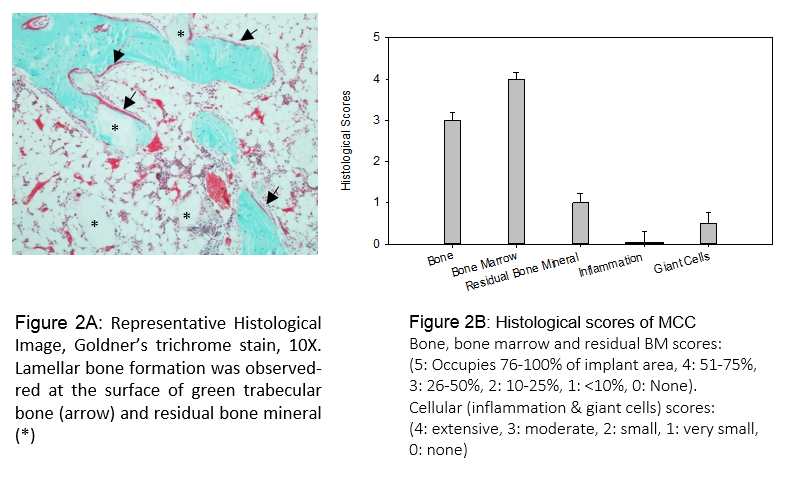
In Vivo Animal Study: Bilateral surgeries were performed on the femoral condyle of each New Zealand White rabbit to create a defect of 5mm in diameter and 6-8mm in depth. MCC scaffolds were implanted, and self-expanded to fully fill the defect site upon hydration by body fluid. At 8 weeks post-surgery, rabbits were euthanized. Specimens were harvested and prepared for histological analysis using a semi-quantitative score system (See Fig. 2B). Parameters scored included the presence of bone and bone marrow, residual implant, inflammation, and giant cell response to the implant.
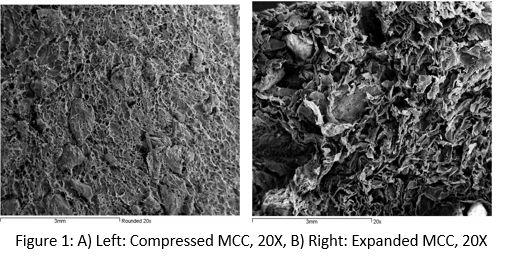
Results and Discussion: In Vitro Characterization: Both the inter-particle distance and the pore size have increased significantly upon expansion (Figs 1A and 1B), providing ample space for cellular infiltration and bone growth. Volume expansion (Table 1) shows a 300% increase in volume from the initial size to the expanded configuration, which is suitable for augmentation of irregular size defects.
In Vivo Animal Study: Histological micrograph (Fig. 2A) showed that lamellar bone formation is present and moderate amount of residual mineral particles was seen at 8 weeks. As expected, the open structure of MCC allowed cellular infiltration for the bone forming process. The low inflammation and giant cells scores indicated no safety concern (Fig. 2B).

Conclusion: The in vitro and in vivo studies demonstrate that the self-expandable MCC implant satisfies its intended function as an osteoconductive scaffold to guide cellular ingrowth and new bone regeneration. The volume expansion characteristic of the MCC is useful to guide the bone growth in defects that have irregular geometries such as tooth extraction sockets and maxilla bone augmentation in sinus membrane elevation procedures in dental surgeries.