Introduction: Injury and disease in the nervous system results in loss of sensory and motor neurons. Tissue engineering strategies have great potential to develop more effective therapeutics by combining appropriate biological cues with responsive biomaterial architectures. We have recently shown that more in vivo-like sensory neuron responses are found in 3D culture vs 2D flat substrates[1],[2]. Unfortunately, primary motor neurons are harder to isolate and culture compared to sensory neurons. Moreover, PC12 cells are a well-characterized sensory neuron-like cell line and provide an alternative to primary sensory neurons. An analogous motor neuron-like cell line, NSC-34, is available but has not yet been characterized for tissue engineering applications. The NSC-34 line was made by the fusion of embryonic mouse spinal cord motor neurons and neuroblastoma and has many properties of primary motor neurons. NSC-34 cells have been used to study neurotoxicity and motor neuron disease, but no information is available on optimum conditions to promote neurite outgrowth or motor neuron-like morphologies. Thus, our goal was to systematically characterize NSC-34 cells and optimize biomaterial microenvironment and culture conditions for tissue engineering applications.
Materials and Methods: Methods were adapted from[1][2]. For 2D studies, we cultured NSC-34 cells on coverslips coated with laminin or type I collagen in DMEM with fetal bovine serum (FBS; 3, 10%), or N2 supplement. For 3D studies, cells were cultured in gels composed of type I collagen with or without laminin or Matrigel in DMEM with 3% FBS. We assessed proliferation via EdU assay. Immunofluorescence reactivity of beta-III tubulin and SMI-32 (a marker of mature motor neurons) was determined and neurites per cell and lengths of neurites were measured.
Results and Discussion: NSC-34 cells exist as proliferating cells with no neurites or as differentiated cells with one or more neurites[3]. We found that serum promoted the proliferating phenotype while N2 promoted neurite outgrowth (Fig 1)[4]. Further, we found that laminin substrates were associated with a higher percent of cells expressing neurites and that the neurites were significantly longer than for collagen substrates, with greatest results at 3% serum (Fig 2). Medium with N2 was associated with lower cell proliferation vs serum-containing medium regardless of substrate. The proliferation results showed low percent proliferation for serum free but no significant difference between collagen and laminin. Preliminary results in 3D gels indicate similar results in that laminin provides a favorable environment for neuronal viability. Ongoing work focuses on rigorously characterizing neuronal morphologies and neurite lengths in 3D cultures.
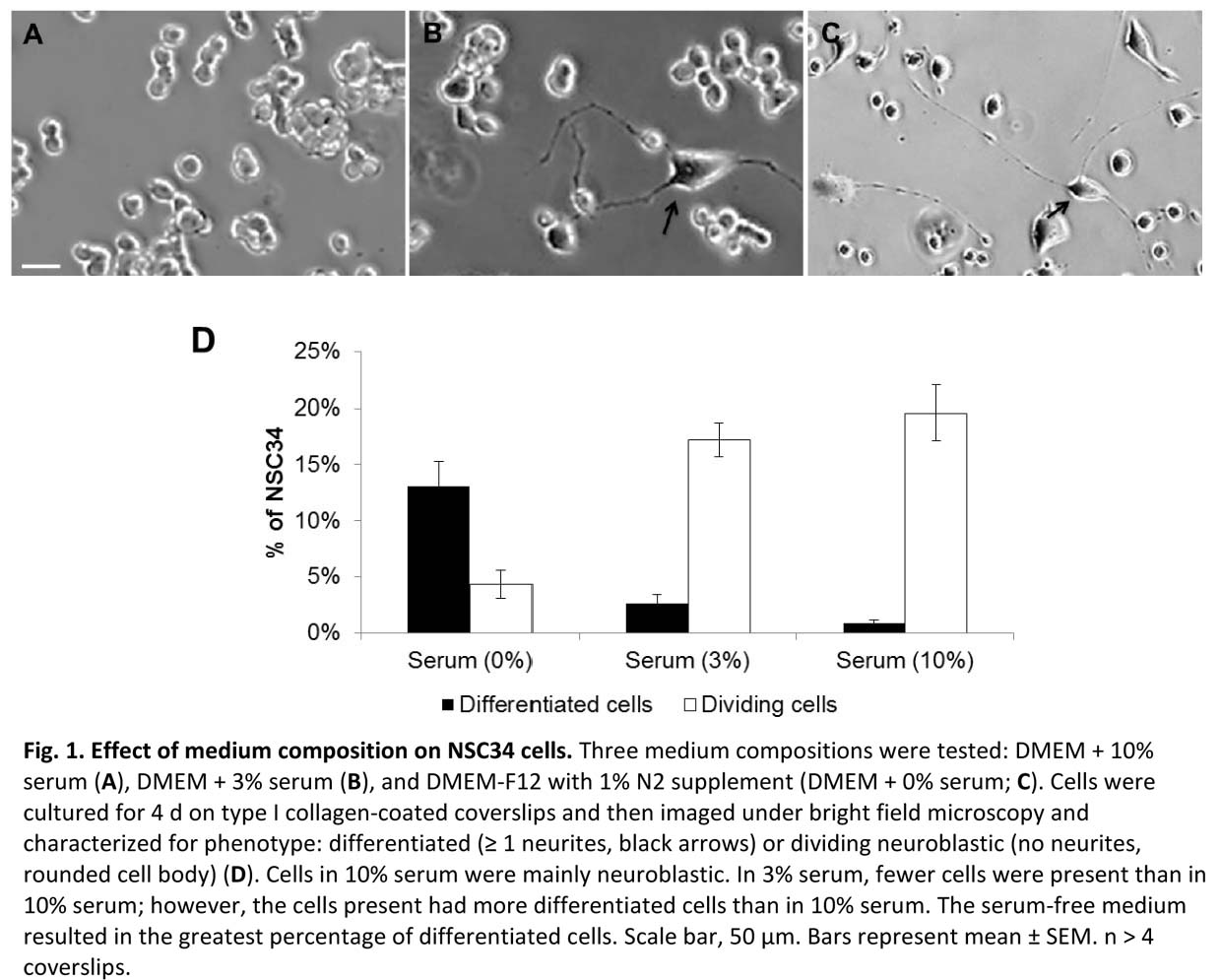
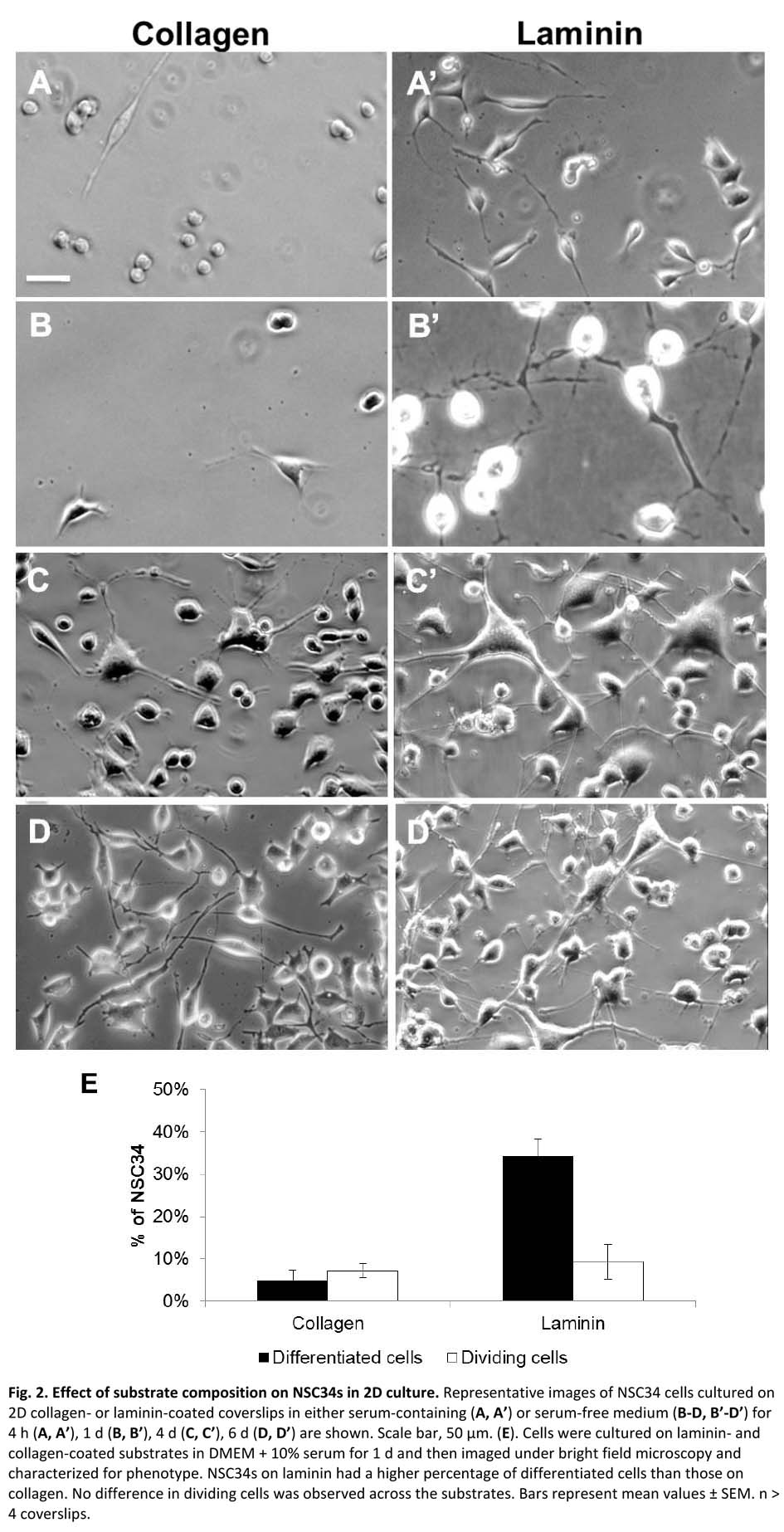
References:
[1] Ribeiro A, S Vargo, E Powell, JB Leach. (2012) Substrate three-dimensionality induces elemental morphological transformation of sensory neurons on a physiologic timescale. Tissue Engineering 18:93-102.
[2] Ribeiro A, S Balasubramanian, D Hughes, S Vargo, E Powell, JB Leach. (2013) β1-integrin Cytoskeletal Signaling Regulates Sensory Neuron Response to Matrix Dimensionality. Neuroscience 248:67-78.
[3] Balasubramanian S, L Walker, E Adamson, JB Leach. Baseline phenotypic characterization of NSC-34 motor neuron cell line for use in tissue engineering applications. To be submitted to Biotechnology and Bioengineering.
[4] Cashman N, et al. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992 Jul. 194(3):209-221.