Introduction: Numerous strategies have been developed to accelerate endothelialization of small diameter vascular grafts. REDV is a fibronectin-derived peptide effective in inducing selective endothelial cell (EC) adhesion. Nitric oxide (NO) stimulates EC proliferation and inhibits smooth muscle cell (SMC) overactivity. Materials containing catalysts such as selenium compounds could generate a sustained in situ flow of NO from endogenous NO donors in the blood. This work constructed a novel vascular graft with catalytic NO generation and promoted EC adhesion for rapid in situ endothelialization.
Materials and Methods:
Preparation: electrospun polycaprolactone (PCL) substrates were modified via layer-by-layer self-assembly to introduce organoselenium immobilized polyethyleneimine (SePEI) as an NO donor catalyst and REDV immobilized hyaluronic acid (HA-REDV).
Groups: PCL, PCLS (10 SePEI/HA bilayers deposited), PCLSR (9 SePEI/HA bilayers plus a SePEI/HA-REDV bilayer).
Catalytic NO generation: S-nitrosoglutathione (GSNO) solution was added onto the materials and accumulated NO generation was measured using a Griess Assay Kit.
Cell culture: ECs alone or ECs/SMCs were seeded with GSNO and incubated for 4 h.
In situ implantation: grafts were implanted into the abdominal aorta of Wistar rats for 2 and 4 weeks.
Results: PCLS and PCLSR materials had a potent capability to accelerate the release of NO from GSNO.
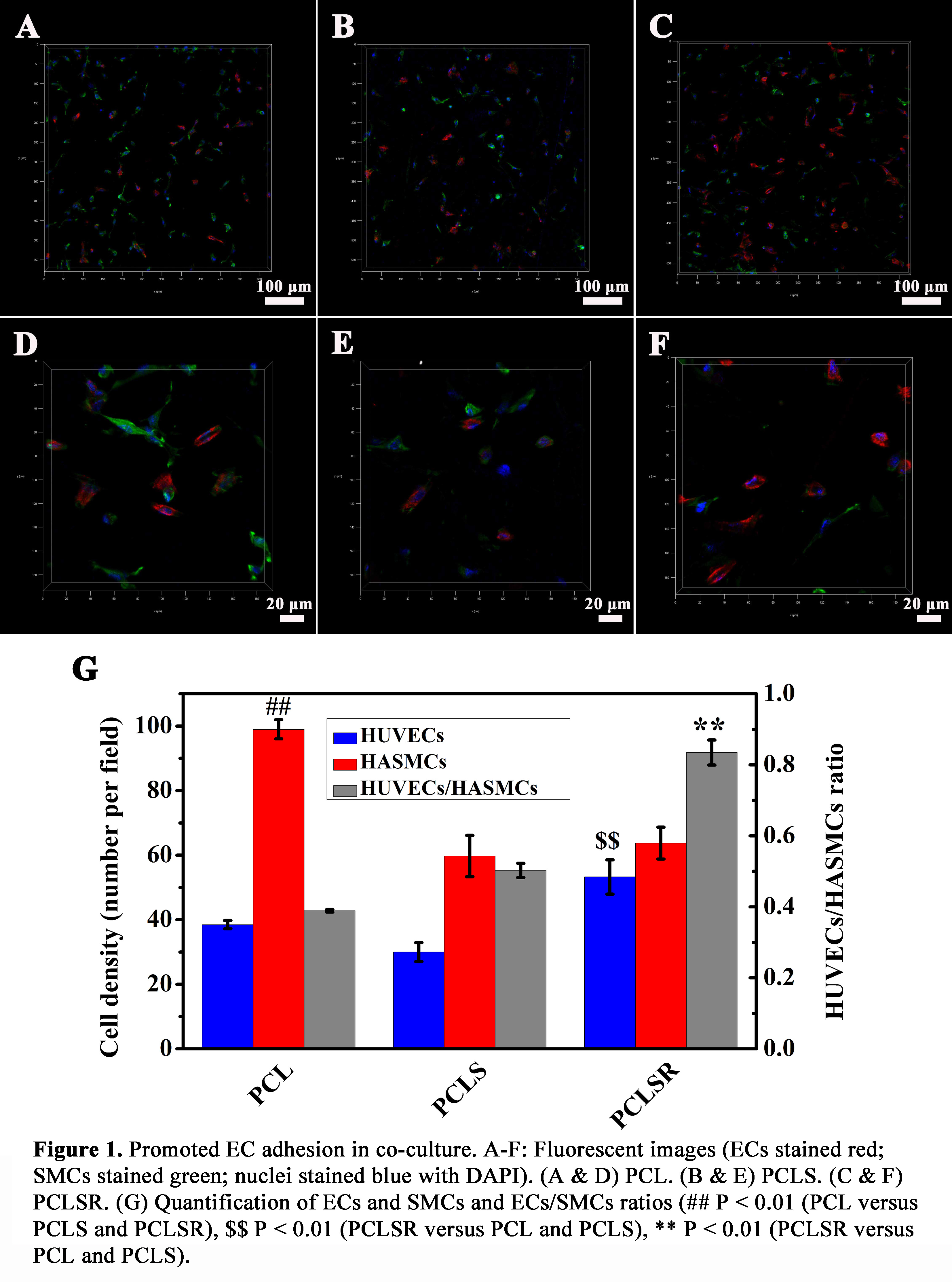
EC adhesion was higher on PCLS films and further increased on PCLSR films. In co-culture, SMC adhesion and spread were significantly inhibited on PCLS and PCLSR films, while EC adhesion was enhanced on PCLSR films. The synergetic effect of NO and REDV increased the ECs/SMCs ratio.
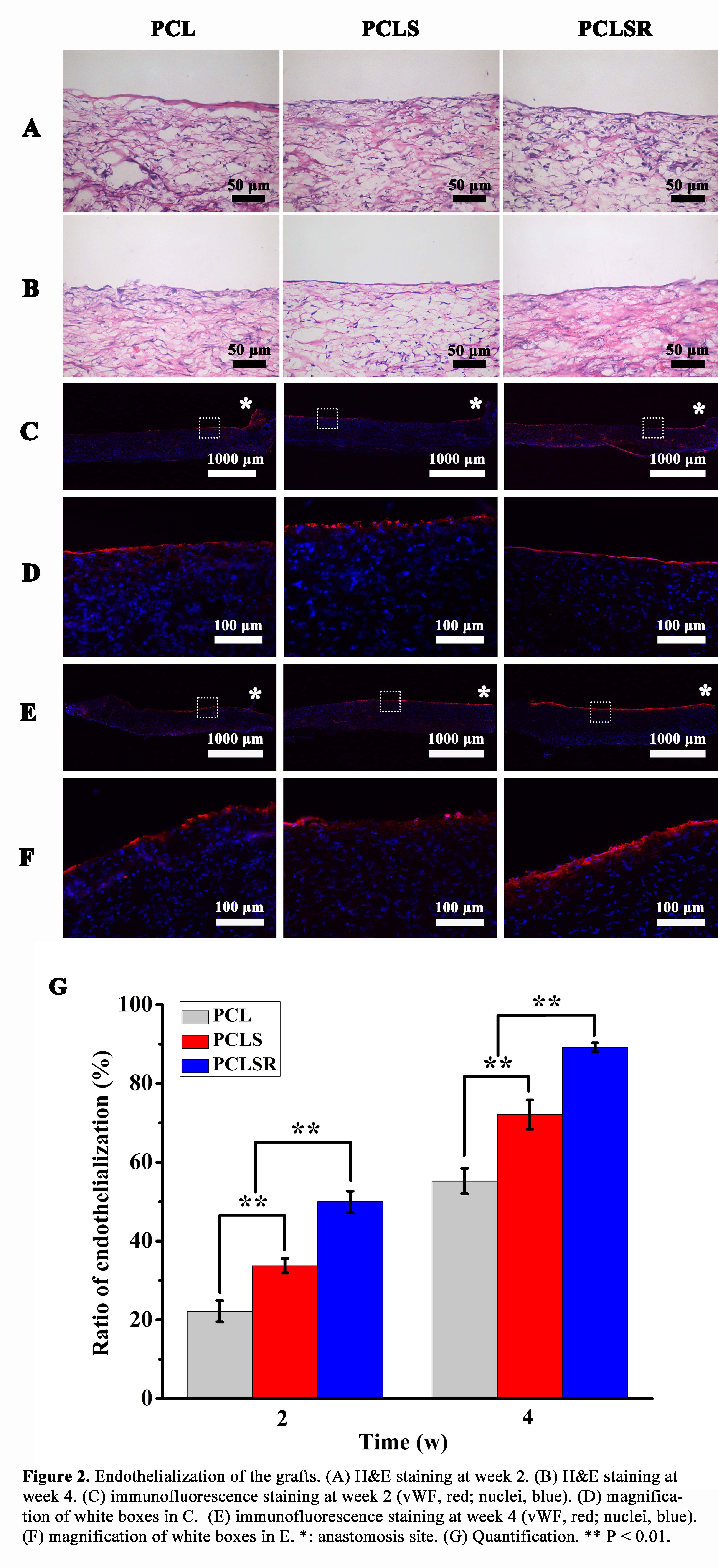
PCLS grafts had a larger endothelial coverage than PCL grafts, and PCLSR grafts showed the most rapid endothelialization. ECs were randomly oriented on PCL and PCLS grafts, but aligned along the blood stream on PCLSR grafts in a pattern similar to the native vessel.
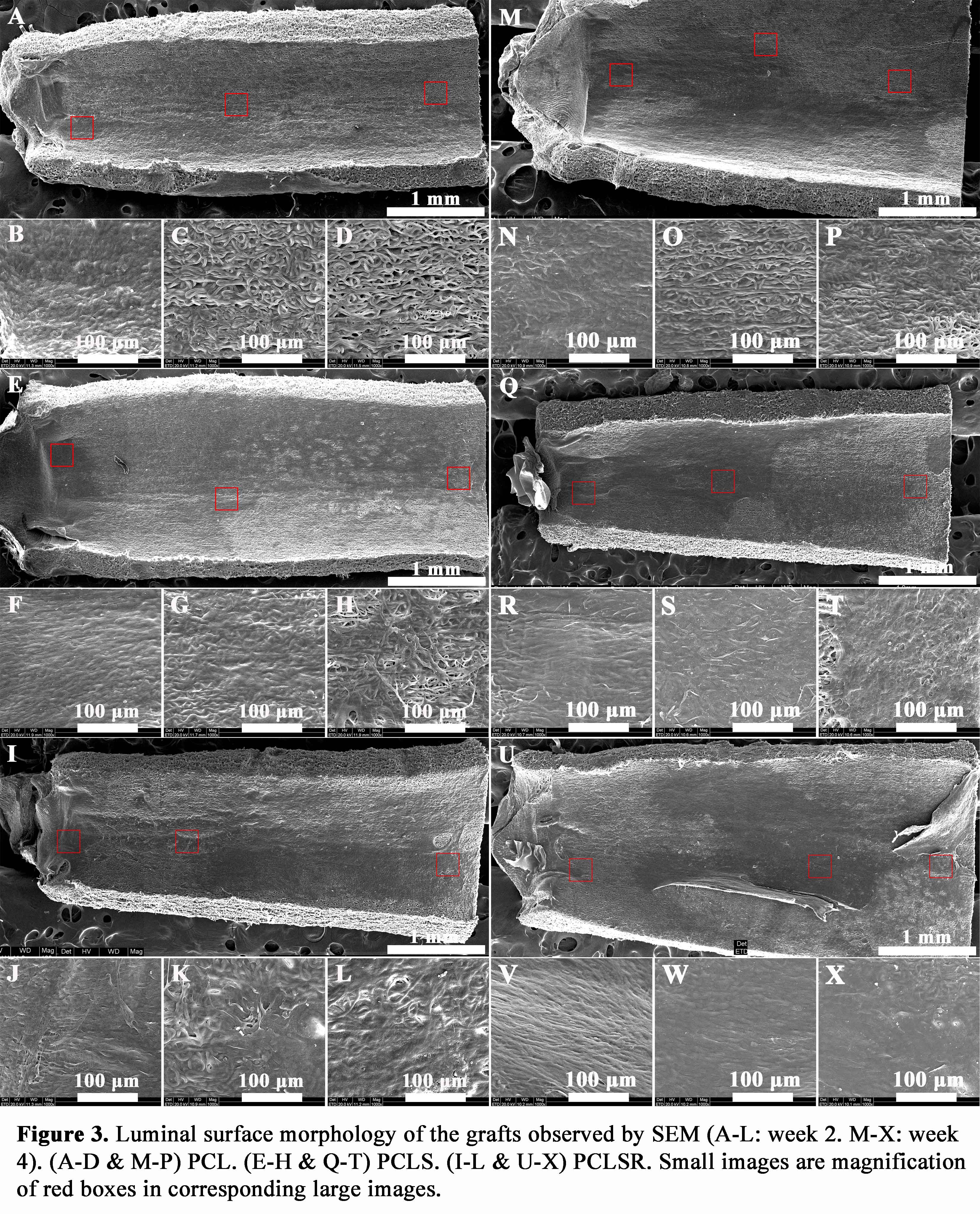
Discussion: In situ catalytic NO generation could eliminate cruxes of NO-releasing materials (limited storage, burst release, and toxicity caused by leakage). Materials constructed in this study are expected to generate a sustained and stable flow of NO when contacting the blood.
Nonselective peptides might mediate the adhesion of various cells and hinder endothelium formation. Hence special attention should be paid to promote competitive EC adhesion. Materials constructed in this study possess great potentials in enhancing endothelialization and preventing intimal hyperplasia.
Conclusion: This work examined the in vivo performance of an NO-generating vascular graft for the first time. The dual modified graft proved efficient in promoting EC adhesion and in situ endothelialization. The appraoch proposed herein may improve current strategies for rapid endothelialization and long-term patency of small diameter vascular grafts.
National Key Basic Research Program of China (“973” Program; grant 2012CB725204); National Natural Science Foundation of China (grant 51073081); PCSIRT (No. IRT13023); Tianjin Natural Science Foundation (grants 13JCYBJC24900 and 13JCZDJC27800)