Introduction: Bisphosphonates (BPs) are recognized as potent inhibitors of osteoclast activity and widely used clinically for the treatment of diseases associated with conditions of bone loss. It is known that BPs act directly on osteoclasts and interfere with specific intracellular biochemical processes such as isoprenoid biosynthesis and subsequent protein prenylation to inhibit cell activity [1]. The ability to bind to bone mineral is the key of their pharmacological action as well as of the efficacy and action duration. The mineral affinity and activity of individual BPs depend on the two covalently bound sidechains, R1 and R2, attached to the central carbon atom (Scheme 1). It has been reported that zoledronate (Zol) displays a greater binding affinity than alendronate (Al) towards apatite (HA) surface [2]. Due to its important role as precursor of biological HA, octacalciumphosphate (OCP) is a good candidate for the preparation of composites for bone tissue repair. In this work we modified the classical procedure of OCP synthesis in order to prepare OCP-Al and OCP-Zol crystals at different concentration of the BPs and we demonstrate the higher affinity of Al for OCP.
Materials and Methods: The synthesis of OCP, in the presence of Al and Zol, was carried out by dropwise addition of a Ca(CH3COO)2 solution into a phosphate solution. The reaction was carried out at 70°C under mechanical stirring. The precipitate was stored in contact with the mother solution for 30 min before dropwise addition of the bisphosphonate solution. Finally the precipitate was filtered, repeatedly washed with distilled water and dried. Zol and Al concentrations were in the range from 0.1 to 1.2 mM. The powders were characterized by X-Ray and SEM analysis, whereas BPs content was determined spectrophotometrically via complex formation with Fe(III) ions.
Results and Discussion: By using both BPs we obtained OCP as the unique crystalline phase, as confirmed by the results of XRD analysis. Al and Zol content in the solid products increased on increasing their concentration in solution up to about 5.5% and 3.5%, respectively. Al and Zol presence during the OCP synthesis caused a reduction of the mean length of the coherently scattering domains and a reduction in the dimensions of the composite crystals compared to those of pure OCP. The data suggest different mechanisms of interaction of Zol and Al with OCP structure, which can be justified by the results of the study of calcium coordination environment.
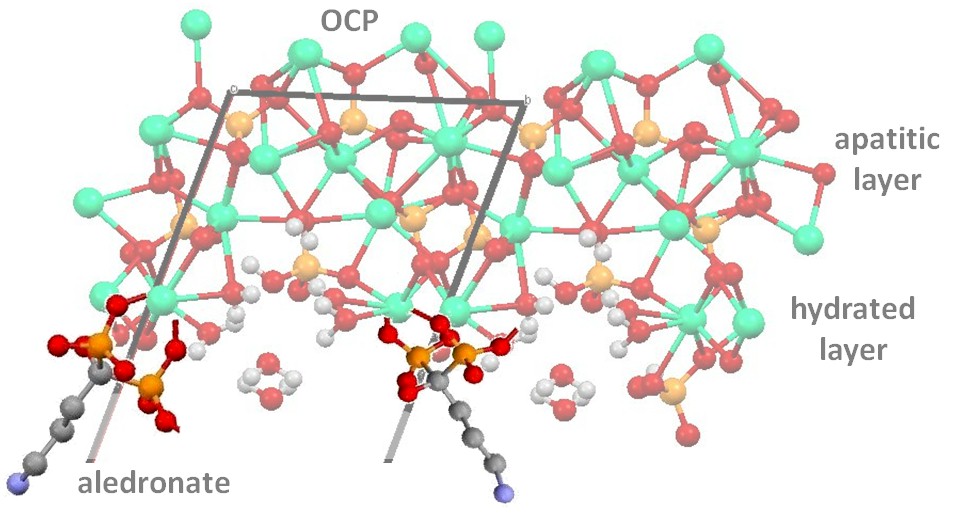
Figure 1: possible interaction of Al with the hydrated layer of OCP structure
Conclusions: The different behavior of Zol and Al towards incorporation into OCP, with respect to that previously observed for HA [3],[4] suggests different mechanisms of interaction between the BPs and the calcium phosphates in the composites, which provide materials with tunable antiresorptive properties and promising potential orthopedic applications.
References:
[1] Russell RGG, Watts NB, Ebetino FH, Rogers MJ, Osteoporos. Int. 2008,19, 733-759.
[2] Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RGG, Ebetino FH, Bone 2006, 38, 617-627.
[3] Boanini E, Torricelli P, Gazzano M, Fini M , Bigi A, Biomaterials 2012, 33, 722-730.
[4] Boanini E, Gazzano M, Rubini K, Bigi A, Adv. Mater. 2007, 19, 2499-2509.