Introduction: Growth factors (GFs) offer the potential to regulate tissue formation, maintenance or regeneration. These biomolecules as powerful signalling tools control cellular behavior, such as adhesion, proliferation, migration and differentiation.GFs are naturally occurring substances secreted by cells; to transfer signals between the cells and their micro environment, by binding and forming stable complexes to their receptors present on the cell surface. Since these molecules mostly are proteins, it is important to retain their biological activity upon inclusion of GFs into these materials and their optimized accessibility to obtain an efficient cell response. By inspiration from the nature, it is possible to incorporate extracellular matrix (ECM) to confine GFs mobility and control their temporal and special delivery. To this aim, different strategies have been used, including chemical or physical surface immobilization. Heparin sulfate; a sulfated, linear, unbranched polysaccharide, structurally composed of disaccharide repeated units, is present in the ECM. This macromolecule has many carboxyl groups which can be served for covalent reaction with surface amine groups of biomaterials[1]. In considering the above discussion, in the present study, it was decided to covalently graft heparin solfate onto the amin functionalized surface and then load VEGF into heparin chains to promote angiogenesis in vitro tissue culture system.
Materials and Methods: The detailed fabrication and surface characterization of amin functionlized fibers have been previously reported[2]. Here, the immobilization of heparin was performed with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and N-hydroxysuccinimide (NHS onto the surface of 100-μm diameter poly (ethylene terephthalate) (PET) monofilaments, via an n-hepthylamine plasma polymer (HApp) bearing amine groups. The successful grafting onto the surface was confirmed by X- ray photoelecron spectroscopy analysis. Then VEGF were physically immobilized onto heparinized polymer fibers, as illustrated in Figure 1. During the entire process, fibers were maintained parallel in a Teflon holder ring (Figure 2 A). HUVECs were then seeded and grown on fibers for confluence. The holder was then embedded in a fibrin gel and culture medium added and the samples were incubated for 20 days. Cells on the fibers were stained for actin filaments, using TRITC–phalloidin, and counterstained with Sytoxgreen to highlight the position of the nuclei subsequently.

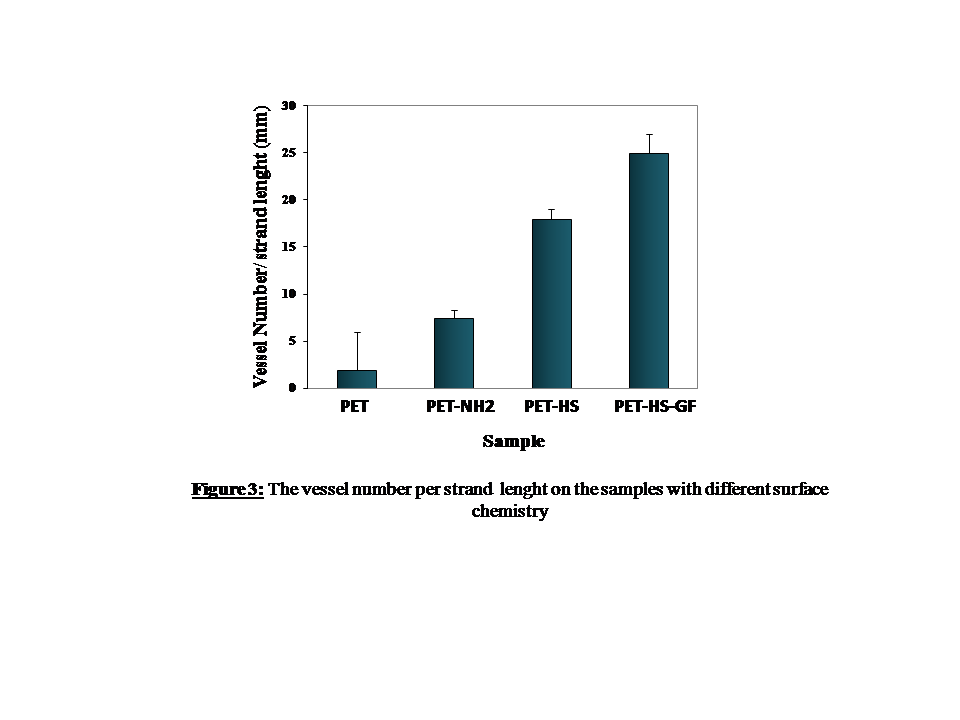
Results and Discussion: The success grafting of the heparin onto the surface was confirmed by X- ray photoelecron spectroscopy analysis, showing the appearance of the sulfur element of about 4 % comparing to uncoated sample for which the sulfur was 0%. VEGF release was evaluated indirectly by cell culture experiment. As shown in figure 2 and 3, tube like structure formation occurred on the HUVECs seeded fibers embedded in the fibrin gel 3D system. Cell strands formed along the fibers and then branches were occurred from those strands forming vascular network like structures (figure 2B and C). The vessel formation on the VEGF loaded sample was higher than others as counted after 20 days of incubation (figure 3). Heparin mediated physically loaded VEGF onto the scaffolding biomaterials can release and promote angiogenesis in vitro tissue culture systems.

Conclusion: Heparin mediated physically loaded VEGF onto the scaffolding biomaterials can release and promote angiogenesis in vitro tissue culture systems. It can be concluded that, VEGF containing HUVECs can stimulate directional micro-vessel network formation within a 3-D environment, such as a fibrin gel.
References:
[1] Harper, S.J., Bates, D.O., The key to anti-angiogenic therapeutics? Nature Reviews Cancer 8, 880-887 (2008).
[2] Cabanas Danes, J., Huskens, J., Jonkheijm, P. Chemical strategies for the presentation and delivery of growth factors. J. Mater. Chem. B, 2014, 2, 2381 (2014).
[3] Hadjizadeh, A., Doillon, C.J., Vermette, P., Bioactive polymer fibers to direct endothelial cell growth in a three-dimensional environment - Biomacromolecules, Biomacromolecules 8 (3), 864-873 (2007).