Introduction: Several FDA guidance documents for benchtop testing of cardiovascular devices have included particulate testing.[1][2] Counting of particulate following simulated use of a medical device can provide an understanding of the possible embolic load during a deployment.
While these documents provide a good solid foundation for performing particle testing, there were no specific details of the actual test method set up. This paper will give an overview of the test method set up that has been used in light of the FDA recommendations.
Materials and Methods: For a summary of the particle test method and considerations for particle testing, two references[3][4] are recommended. The final product for testing was a finished medical device (Devices A, C, and D) along with a device manufactured by another company (Device B). The untreated device was not coated while the remaining devices were coated with a thin, hydrophilic polymer to improve device performance. Two sizes of the device, 24 French (Fr) and 22 Fr, were included in this study. The effect of sterilization was evaluated by testing some of the devices both before and after sterilization (pre-process and post process). Testing was performed in a cleaned horizontal HEPA work bench to minimize background environmental particulate. Water and glassware beakers used for testing were verified to pass the USP 788 criteria.[5] Devices were prepared following their instructions for use (IFU) by flushing the inner lumen and rinsing the outer surface with filtered water. The device was inserted into a tortuous glass model based on geometry of 3D reconstructed CT scans taken from patient anatomy. The glass model was pre-filled with filtered water and the device was inserted as far as possible. The device was then retracted and inserted as necessary to mimic delivery within the patient. The device was left within the model while the filtered water was drained and collected in a cleaned test beaker. Filtered water that was collected in a reservoir above the model was released in order to provide physiological flow conditions. Rinses were repeated to sufficiently remove the particulate from the device and glass model. Finally the device was removed and a final rinse of the glass model was performed. All rinses were deposited into the collection beaker and this combined volume was tested using a light obscuration particle counter following USP 788 testing conditions.
Results and Discussion: The test method was sufficient to differentiate between medical devices as shown in Figure 1. In addition to differentiating between the untreated device and the other devices tested, the test was able to determine a difference before and after sterilization for both sized devices pre and post process. Finally, a trend of lower particle values for the smaller device size was observed. Following particle testing, additional examination of the particles could be performed as recommended by AAMI TIR42:2010 for determining particle origin.[6]
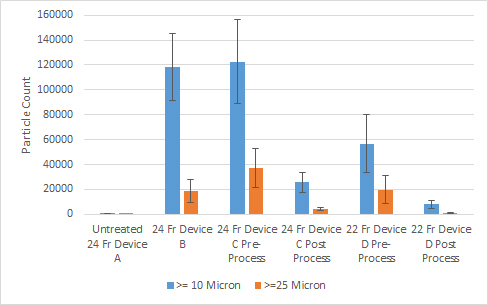
Figure 1 Particle Count Comparison between Cardiovascular Medical Devices
Conclusion: A method for testing a cardiovascular medical device for particulate following simulated use was described that followed FDA guidelines and provided further specifics for the test.
References:
[1] Guidance for Industry and FDA Staff: Non-Clinical Engineering Tests and Recommended Labeling for Intravascular Stents and Associated Delivery Systems, Food and Drug Administration, April 18, 2010
[2] Class II Special Controls Guidance Document for Certain Percutaneous Transluminal Coronary Angioplasty (PTCA) Catheters, Food and Drug Administration, September 8, 2010
[3] S Reynolds and R Lunceford, “Analyzing Particulate Matter on Medical Devices”, Medical Device and Diagnostic Industry, May 2009
[4] ASTM F2743-11, “Standard Guide for Coating Inspection and Acute Particulate Characterization of Coated Drug-Eluting Vascular Stent System”, ASTM International, 2011
[5] General Chapter <788>, “Particulate Matter in Injections”, U.S. Pharmacopeia, 2012
[6] AAMI TIR42:2010, “Evaluation of Particulates Associated with Vascular Medical Devices”, Association for the Advancement of Medical Instrumentation, 2010