Introduction: 3D printing of hydrogels is intensively being explored to enable advanced 3D cell culture under tissue like conditions. Soft hydrogels in the compression modulus range below 1 MPa are required for accurate modeling of soft tissue. However, such mechanically fragile hydrogels are not easily interfaced to much stiffer tubing required in perfusion systems. Thus, both basic cell research studies and system interation may benefit from access to a material and a 3D-compatible processing method where the mechanical properties can be tuned locally from very soft to medium hard during fabrication.
Light-guided 3D printing, e.g. stereolithography, is a promising method class for high-resolution shaping of soft and hard materials. However, no current material and method can vary the hardness broadly within a printed object without complex transfer between multiple pre-material baths for each layer printed. This limination is overcome by combining spectrally independent radical-initiated photopolymerization of acrylates (soft) and cation-initiated polymerization of epoxies (hard). The local compression moduli of the resulting material are fully definable in the range from 100 kPa – 10 MPa by illuminating at two wavelengths (365 nm and 450 nm) for specific times.
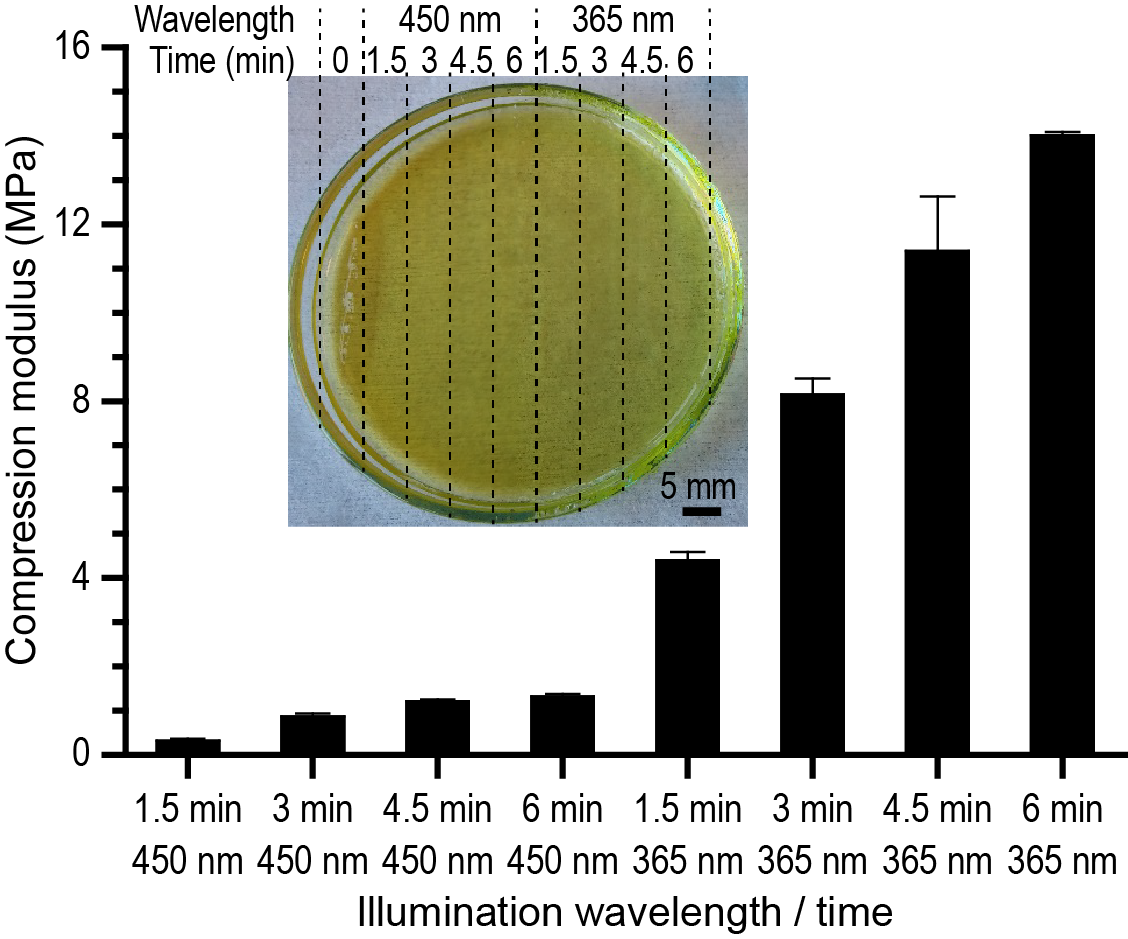
Materials and Methods: Poly(ethylene glycol)-diacrylate 700 Da (PEGDA) and 3,4-epoxycyclohexylmethyl 3,4-epoxycyclohexanecarboxylate (ECC) was mixed in diethylene glycol diethyl ether (DGDE) with added camphorquinone and triarylsulfonium hexafluoroantimonate photoinitiators. Light exposure was by patterned light at 450 nm (LightCrafter 4500), masked light at 365 nm (MA4, Karl Süss), or using custom-built micro-stereolithography system with simultaneous exposure at 365 and 450 nm. The solvent in the final hydrogels was removed by immersion in ethanol and in water. Compression analysis used a Texture Analyzer (Texture Technologies) with a Ø2 mm probe. The probe size required stepped gradients, enabled by manually exposing progressively larger areas of the prepolymer solution in a Ø60 mm petri dish.
Results and Discussion: The broadest range of compression moduli could be obtained from a solution of 20 %w/v PEGDA and 40 %w/v EEC. Figure 1 shows the result from repeated stepped exposure of a prepolymer solution. The inset displays a photo of the Ø6 cm petri disk with the exposed hydrogel. Both the surface and the bulk of the gel appeared continuous. This continuous gel spans compression moduli from approx. 300 kPa to 14 MPa. Other prepolymer mixtures resulted in moduli below 100 kPa or above 20 MPa, at the cost of a smaller dynamic range. Preliminary experiments using a custom-built dual-color micro-stereolithography system indicate the possibility for mechanical gradients on the length scale of tens of microns. The hydrogel cytocompatibility was assessed by culturing HT29 cells for 2 days and analyze their viability (MTS, TCPS as reference). Viability correlated strongly with the hardness, with 10-20% of the control on the soft cell-repellent PEGDA-rich gels and 70-80% of the control on the hardest epoxy-rich gels.
Conclusion: The use of largely uncoupled light-induced initiation mechanisms in mixtures of commercially available reagents provides an easy path to locally tune the mechanical properties using multi-colored light shaping of cell compatible hydrogels.