We used two types of biodegradable and photo-crosslinkable polymers, poly(ε-caprolactone) triacrylate (PCLTA) and poly(ethylene glycol) diacrylate (PEGDA), with different molecular weights to demonstrate the roles of mechanical and chemical factors in regulating nerve cell behavior. Rat Schwann cell precursor line (SpL201) cells were found to proliferate more and differentiate better on stiffer substrates of both networks but differences existed between these two networks because of surface chemistry.
Three PCLTAs and three PEGDAs were synthesized previously in our lab[1],[2]. PCLTA2k, 5k, and 7k had number-average molecular weights (Mns) of 2220, 5400, 6700 g mol-1, respectively. PEGDA1k, 3k, and 10k had Mns of 1450, 5450, 14500 g mol-1 respectively[1],[2]. Rat SpL201 cells were seeded onto 70% alcohol-sterilized disks of crosslinked PCLTAs and PEGDA hydrogels at a density of ~15,000 cells per cm2 for 1, 4, and 7 days. SpL201 differentiation was also conducted after cells attached onto the substrates. The growth medium was replaced by the differentiation medium. All attached cells were fixed at day 7 and stained with primary antibody of mouse monoclonal anti-oligodendrocyte marker O4 antibody. Cells were counter-stained with DAPI at room temperature for photographing.
All the networks were amorphous at 37°C and therefore the mechanical properties of these networks were determined by crosslinking density. G' decreased from 1400 kPa for crosslinked PCLTA2k to 650 and 240 kPa for crosslinked PCLTA5k and 7k, respectively. PEGDA hydrogels showed the same trend in G'. Crosslinked PEGDA1k had G' of 260 kPa. It decreased to 76 and 12 kPa for crosslinked PEGDA3k and 10k, respectively. As shown in Figure 1, the number of SpL201 cells decreased from PCLTA2k to 7k and from hydrogels of PEGDA1k to 10k at all time points, indicating better proliferation on stiffer surfaces for both hydrophobic PCLTA networks and hydrophilic PEGDA hydrogels. However, SpL201 cells proliferated much slower on crosslinked PCLTA7k than PEGDA1k hydrogels, when they had similar mechanical properties. It suggested that surface chemistry and wettability of the networks was also important in controlling cell proliferation besides mechanical properties. SpL201 cell differentiation upon forskolin treatment was quantified using O4 marker at day 7. As shown in fluorescence images in Figure 2, more cells are stained with O4 on the stiffer substrates for both PCLTA and PEGDA networks. Normalized cell differentiated area shows the same trend that larger areas have upregulated O4 marker on stiffer substrates. These results suggested that stiffer substrates support SpL201 to upregulate O4 marker toward cell maturation.
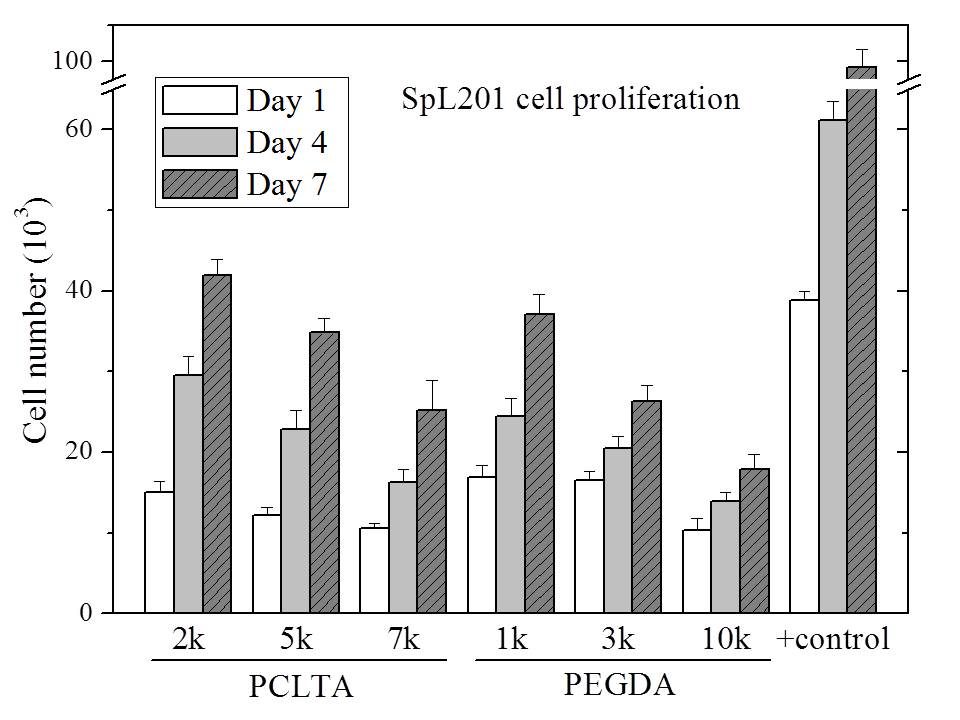
Figure 1. Rat SpL201 cell proliferation on crosslinked PCLTA disks and PEGDA hydrogels at days 1, 4, and 7 post-seeding, compared with cell-seeded tissue culture polystyrene (TCPS) as positive control. p < 0.05 between two neighboring groups at each time point.
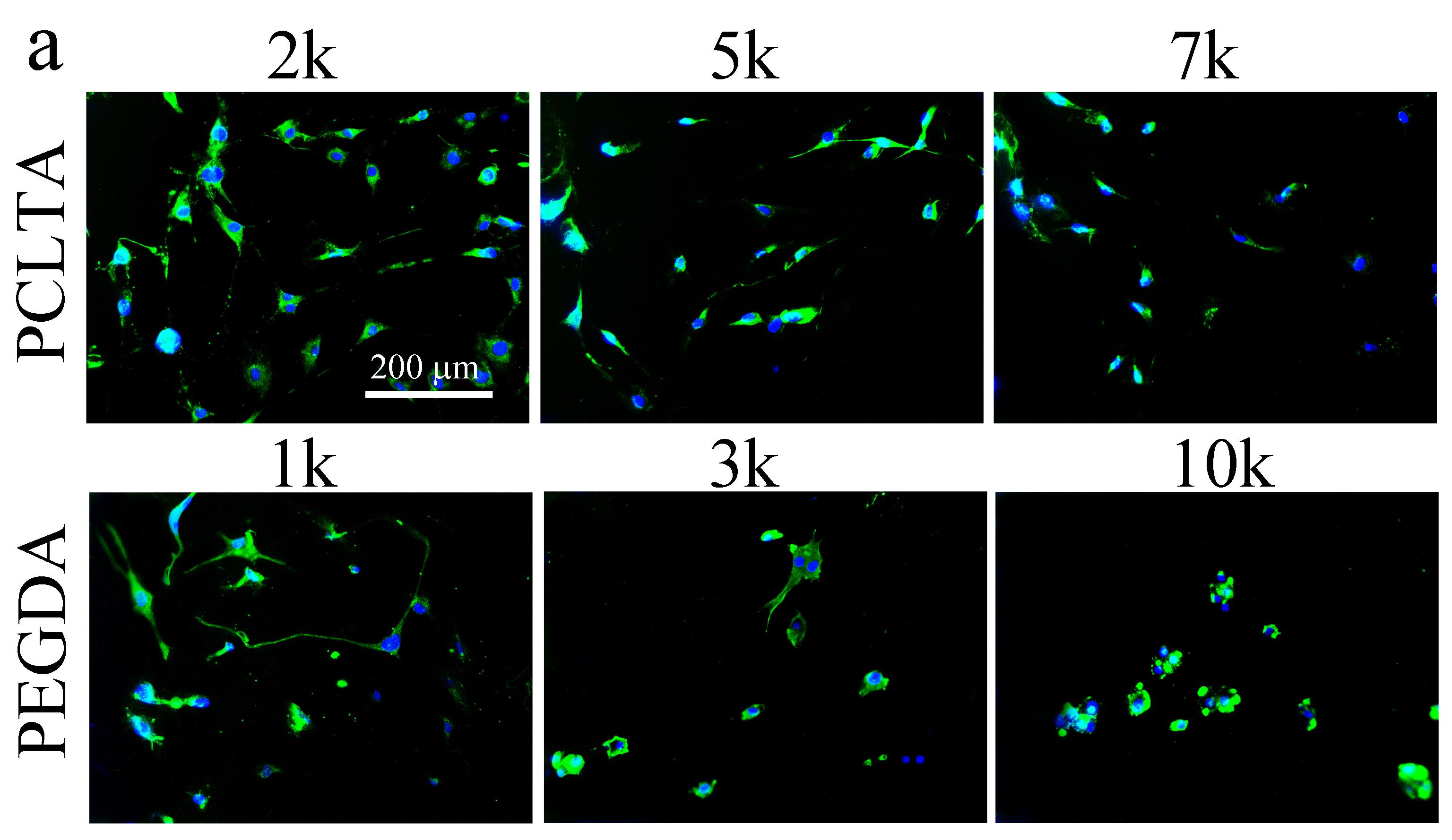
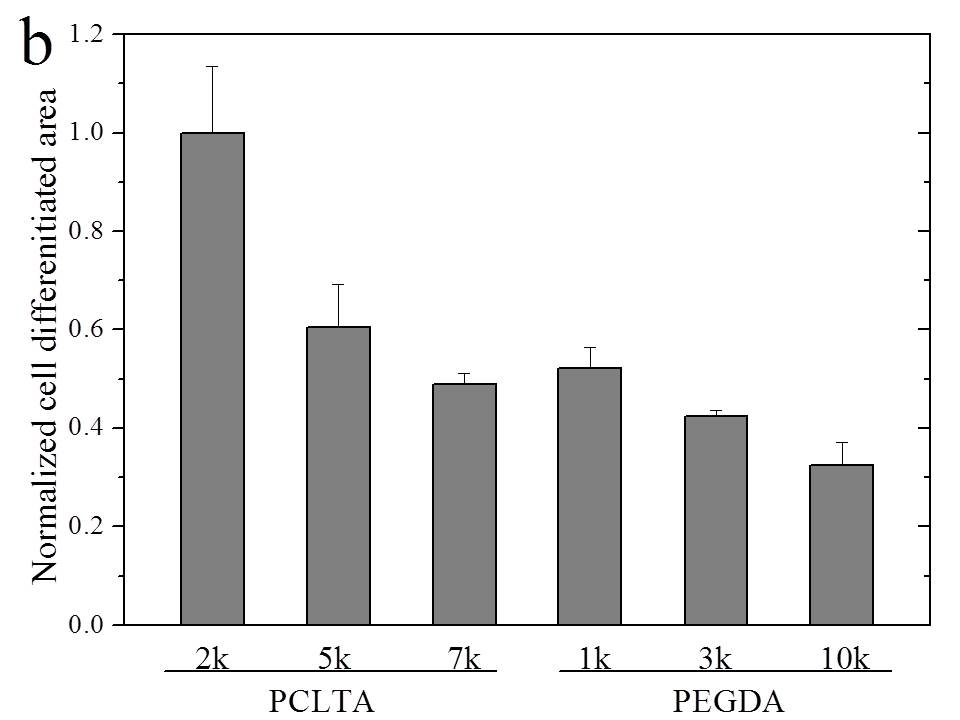
Figure 2. (a) Fluorescence cell images for differentiated SpL201 cells stained with O4 marker (green) and DAPI (blue) at day 7 on the disks of PCLTA networks and PEGDA hydrogels. Scale bar of 200 µm is applicable to all. (b) Cell differentiated area of O4 marker on all the substrates normalized to the value on PCLTA2k. p < 0.05 between two neighboring groups.
Conclusions: We have used three hydrophobic networks of PCLTA and three PEGDA hydrogels to investigate the chemical and mechanical factors in cell-material interactions. After photo-crosslinking, both PCLTA and PEGDA with different molecular weights demonstrated well-controlled shear moduli from to 1400 to 10 kPa. The role of mechanical properties was demonstrated by rat SpL201 proliferation which was better supported on stiffer substrate of both networks. Chemical factors were indicated by lower cell number on hydrophobic PCLTA network than PEGDA hydrogels when their mechanical properties were similar. SpL201 cell differentiation showed the same trend as stiffer substrates could better promote upregulation of O4 marker toward cell maturation. The present results are beneficial for designing optimal nerve conduits using PCLTA as tube materials and PEGDA as hydrogel fillers for peripheral nerve regeneration.
NSF (DMR-11-06142 and 15-07977).
References:
[1] Cai L. Polymer 2010, 51, 164-177.
[2] Cai L. Biomacromolecules 2012, 13, 342-349.