Introduction: Physical trauma or ischemia results in significant damage to the central nervous system (CNS). The regenerative capacity of the injured adult CNS is extremely limited, due to both extrinsic microenvironmental factors and intrinsic, age-related changes in neuronal biochemistry. Many studies have shown that diverse extracellular inhibitors of neuroplasticity including both MAIs (Myelin associated inhibitors) and CSPGs (Chondroitin sulfate proteoglycans) may act through common intracellular signaling pathways. Neurite growth inhibition in response to MAIs and CSPGs has been shown to be associated with activation of RhoA and Rho kinase (ROCK) and can be overcome by Rho/ROCK inhibitors. The long term goal of our work is to develop neuron-specific multi-functional polymeric micelle nanotherapeutics for combinatorial delivery of multiple bioactive molecules targeting different barriers to plasticity and axonal regeneration. To achieve this goal, we designed amphiphilic copolymers (poly(lactide-co-glycolide)–g-polyethylen imine : PgP) as a drug and nucleic acid delivery carrier. Here, we show that PgP micelle is capable of efficiently knockdown the RhoA gene expression in B35 cells in presence of 10% serum in vitro and in the rat compression spinal cord injury model in vivo.
Materials and Methods: Poly (lactide-co-glycolide)-graft-polyethylenimine (PgP) was synthesized and characterized by 1H-NMR/GPC. PgP/Rho A siRNA were prepared at various (N/P) ratios ranging from 5/1 to 30/1. B35 cells were transfected with various PgP/RhoA siRNA in media containing 10% serum and total RNA was isolated at 72hrs post-transfection. The efficiency of Rho A gene knockdown was measured by real-time PCR using the QuantiTect SyberGreen PCR kit (Qiagen) with RhoA gene -specific primers. Relative changes in gene expression levels will be analyzed by the delta-delta CT method. Laminectomy was performed on the back of Sprague Dawley rats and the T9 spinal cord region was exposed. PgP/RhoA siRNA polyplexes (20 µg RhoA siRNA) at various N/P ratio were prepared and injected at the T9 spinal cord region. At 7 days after polyplex injection, the rats were sacrificed and spinal cord sites were retrieved and total RNA was isolated and real-time PCR were performed as described above. To evaluate the RhoA knockdown in protein level, animals were sacrificed via cardiac perfusion with 4% paraformaldehyde solution. The retrieved spinal cords were fixed and sectioned and stained with fluorescence dye conjugated RhoA antibody.
Results: PgP molecular weight was determined approximately 37,000 by GPC. At 3 days after transfection of PgP/Rho A siRNA polyplexes in B35 cells, rho A gene expression was reduced at all N/P ratio with PgP/RhoA siRNA and achieved ~50% knockdown at N/P ratio of 30/1, while PEI/Rho A siRNA showed a~10% knockdown. To evaluate silencing efficiency of PgP/Rho siRNA polyplexes in vivo, we injected polyplex in spinal cord injury site. RhoA expression at 7 days post-injury was significantly increased (~2.5-fold) relative to the sham group (Fig 1).

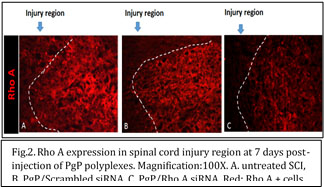
At 7 days post-injection of PgP/RhoA siRNA polyplexes in spinal cord injury region, Rho A gene expression was significantly reduced in all polyplex-treated animal groups compare to untreated SCI group. At 7 days post-injection, the treatment of PgP/RhoA siRNA complexes showed the less RhoA positive area compared with other groups (Fig.2).
Conclusion: These studies demonstrated that PgP is a promising siRNA delivery carrier in B35 cells in vitro and in rat compression spinal cord injury model in vivo. These results suggest that PgP could be useful for therapeutic nucleic acid delivery to treat spinal cord injury. Currently, we are evaluating the utility of PgP for combinatorial drug/nucleic acid delivery.
Research reported in this publication was supported by NIGMS of theNational Institutes of Health under award number 5P20GM103444-07.
References:
[1] Bandtlow et al.,. GLIA 2000; 29: 175-181
[2] Lee et al. Trans SFB p.917 (2010).