Introduction: Although synthetic bioceramics show acceptable results as bone substitutes[1]-[3], biomaterial derived from natural bone material such as autografts and allografts present better performance in vivo. One possible explanation for this difference between natural and synthetic bone substitutes could be the variation in the elemental composition, specifically the presence of trace elements[4],[5].
Ceramics are often composed of a limited amount of elements such as calcium and phosphorous, however, the natural bone contains a much larger variety of trace elements. Many of these elements have been shown to have beneficial effects in bone formation and bone health. Trace elements have been introduced to synthetic ceramics in order to benefit their properties. However, no bioceramic has been produced before with the same elemental composition as the natural bone[6]-[11].
For these reasons, our study was conducted in order to develop biomimetic ceramics that contain the same elemental composition of natural bone. To do so, deproteinized bovine bone ash was incorporated into dicalcium phosphate cement system.
Materials and Methods: Bovine bone ash deproteinization was produced by heating up to 800 oC[12],[13]. The resultant material was characterized using X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR), and compared to a synthetic hydroxyapatite reference (figure 1A). The elemental composition of both materials was determined using inductively coupled plasma (ICP). In order to get biomimetic ceramics, different formulations were tried using bone ash in combination with a source of acidic phosphate group such as phosphoric acid, and monocalcium phosphate monohydrate (MCPM), and citric acid as a reaction modifier to get biomimetic ceramic cements. The derived cements were also characterized using XRD (Figure 1B). Mann-Whitney U test was used to evaluate the significance of elemental differences between both materials (significant difference is considered when p< 0.05).
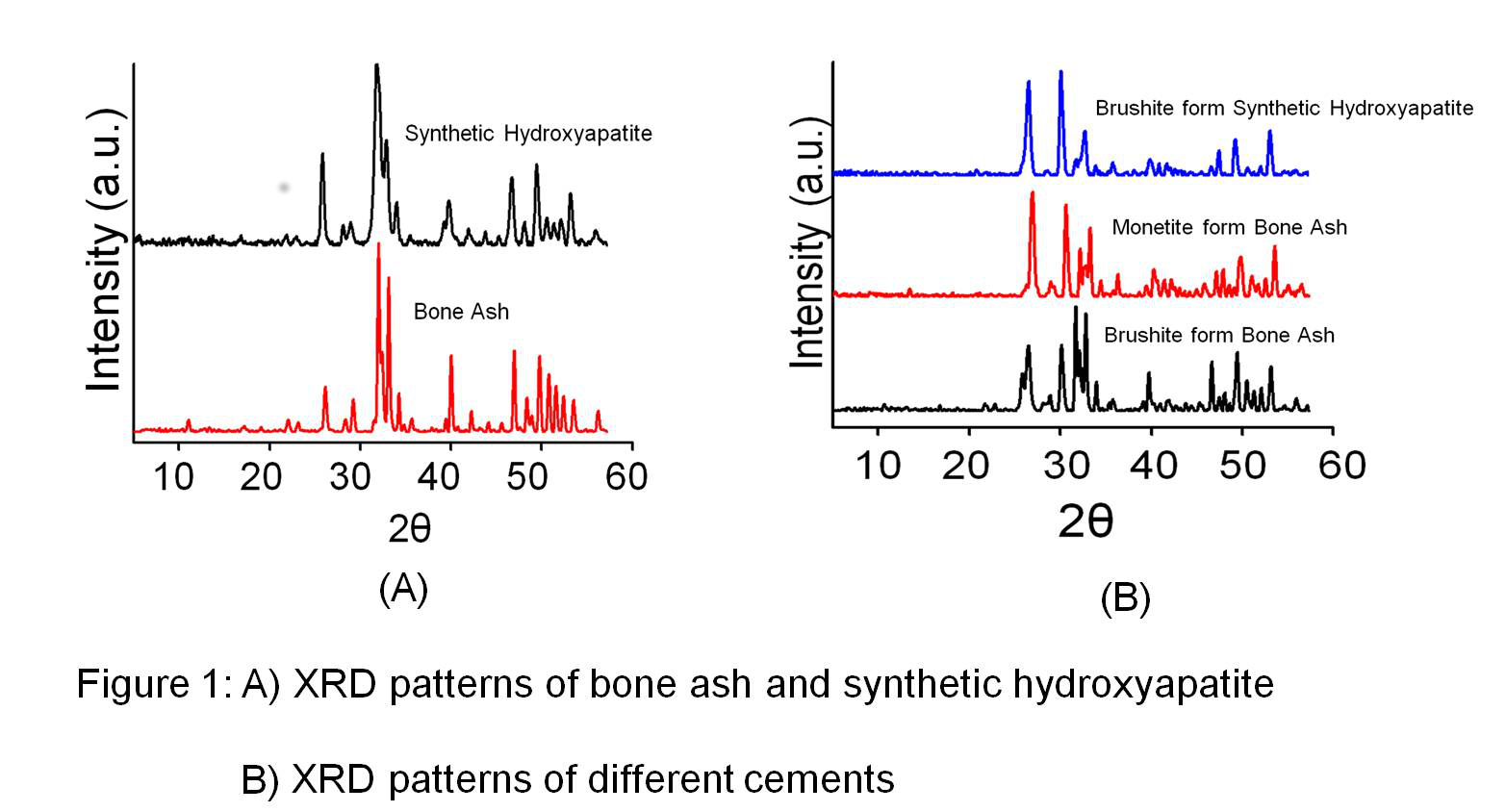
Results and Discussion: Bone ash was made mainly of apatite as the predominant phase, and besides calcium and phosphorus, its elemental composition also included other elements such as magnesium, potassium, sodium, and zinc, which are commonly present in the elemental composition of bone. With bone ash, it was possible to prepare dicalcium phosphate cements of either brushite or monetite by modifying the powder/liquid ratio, the concentration of phosphoric acid and the amount of monocalcium phosphate monohydrate. We noticed that the elemental composition of cements prepared with bone ash was comparable to that of natural bone (Table 1).
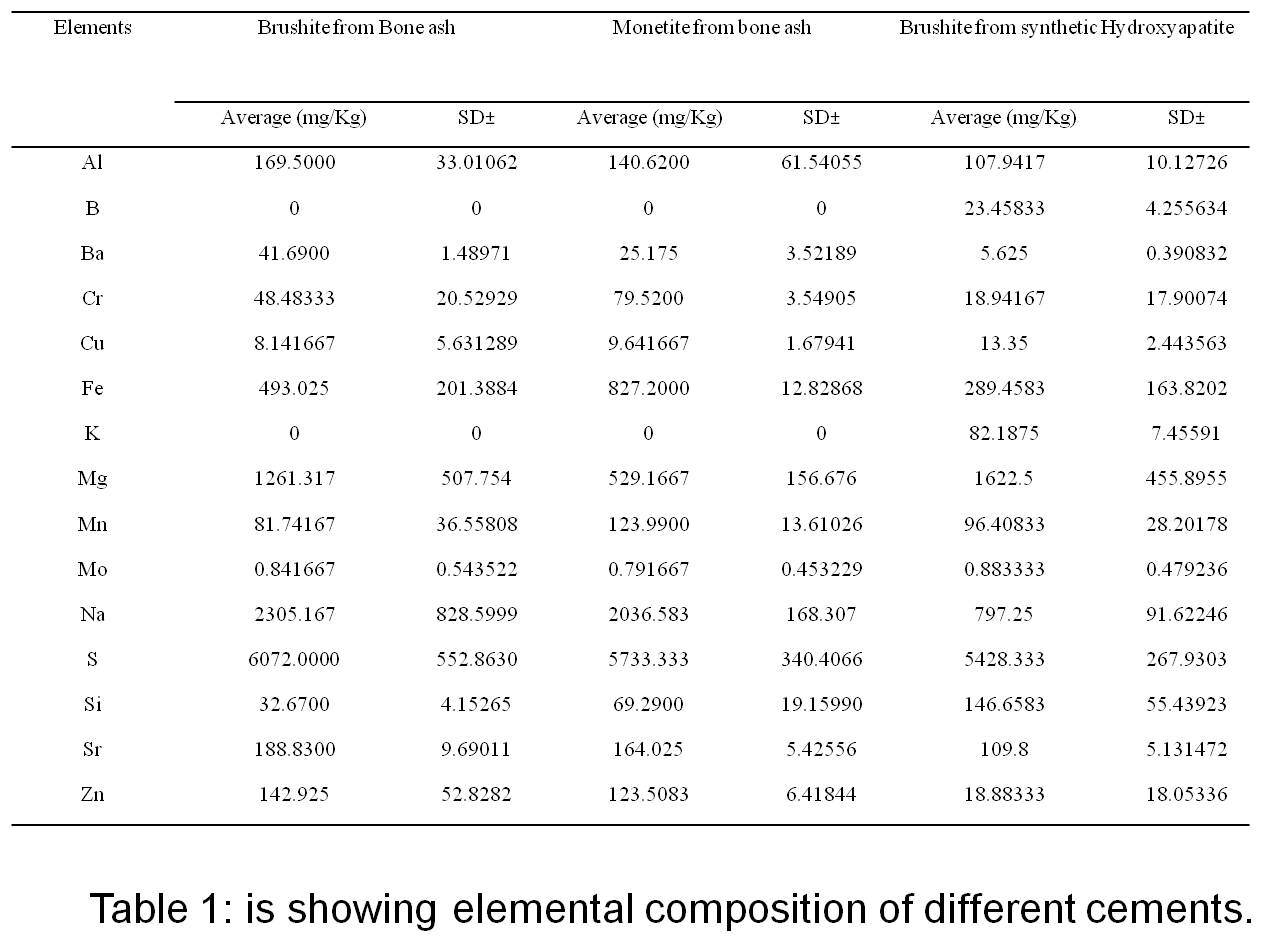
Conclusion: Our findings establish the possibility using natural bovine bone ash to produce cements containing biomimetic concentration of the trace elements found in bone.
References:
[1] Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, et al. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. Journal of materials science Materials in medicine. 2014;25(10):2445-61.
[2] Petite H, Viateau V, Bensaid W, Meunier A, de Pollak C, Bourguignon M, et al. Tissue-engineered bone regeneration. Nat Biotech. 2000;18(9):959-63.
[3] Barrere F, van Blitterswijk CA, de Groot K. Bone regeneration: molecular and cellular interactions with calcium phosphate ceramics. Int J Nanomedicine. 2006;1(3):317-32.
[4] Wu C, Wang J, Li P, Liu G, Li X, Ma H, et al. Bone mineral density and elemental composition of bone tissues in "red-boned" Guishan goats. Biological trace element research. 2012;149(3):340-4.
[5] Bayliss L, Mahoney DJ, Monk P. Normal bone physiology, remodelling and its hormonal regulation. Surgery (Oxford). 2012;30(2):47-53.
[6] Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. Journal of the American College of Nutrition. 2009;28(2):131-41.
[7] Castiglioni S, Cazzaniga A, Albisetti W, Maier JA. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5(8):3022-33.
[8] Sila-Asna M, Bunyaratvej A, Maeda S, Kitaguchi H, Bunyaratavej N. Osteoblast differentiation and bone formation gene expression in strontium-inducing bone marrow mesenchymal stem cell. The Kobe journal of medical sciences. 2007;53(1-2):25-35.
[9] Marie PJ, Hott M, Modrowski D, De Pollak C, Guillemain J, Deloffre P, et al. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1993;8(5):607-15.
[10] Calhoun NR, Smith JC, Jr., Becker KL. The role of zinc in bone metabolism. Clin Orthop Relat Res. 1974(103):212-34.
[11] Barralet J, Gbureck U, Habibovic P, Vorndran E, Gerard C, Doillon CJ. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue engineering Part A. 2009;15(7):1601-9.
[12] Bahrololoom ME, Javidi M, Javadpour S, Ma J. Characterisation of natural hydroxyapatite extracted from bovine cortical bone ash. J Ceram Process Res Journal of Ceramic Processing Research. 2009;10(2):129-38.
[13] Younesi M, Javadpour S, Bahrololoom ME. Effect of Heat Treatment Temperature on Chemical Compositions of Extracted Hydroxyapatite from Bovine Bone Ash. J of Materi Eng and Perform Journal of Materials Engineering and Performance. 2011;20(8):1484-90.