Introduction: Generation of extracellular matrix (ECM) biologic scaffolds for tissue engineering and regenerative medicine applications commonly involves the use of detergents to solubilize cell membranes and dissociate DNA from proteins. The deleterious effects of detergents upon ECM structure, growth factor content and protein ultrastructure are well known. However, the effect of detergent treatment upon the surface ligand landscape of biologic scaffolds has not previously been characterized. The objective of this study was to determine the effect of different detergents upon the surface molecular functionality of a representative tissue, specifically the basement membrane complex (BMC) of porcine urinary bladder.
Methods: The BMC and underlying lamina propria were isolated and harvested from porcine urinary bladders. Six different treatments were utilized comprising five detergents: 3% Triton-X 100, 4% sodium deoxycholate, 1% and 0.1% sodium dodecyl sulfate (SDS) and 8 mM 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) and a non-detergent agent, 0.1% peracetic acid. BMC samples were analyzed by time of flight secondary ion mass spectroscopy (ToF-SIMS), a powerful and sensitive surface analytical technique, to provide information on the composition, structure, orientation and spatial distribution of molecules present on the surface of the treated bladders. Positive and negative ion ToF-SIMS spectra were obtained from 100 x 100 µm spots per treatment group (n≥7 from ≥3 replicate samples) on the basement membrane side with an ION-TOF 5 instrument using a 25 keV Bi3+ analysis beam. Total ion dose was maintained under 1012 ions/cm2. Primary human urothelial cells (HUCs) were seeded upon the basement membrane surface of detergent treated bladders; bladders treated with water only served as a control. HUCs were cultured for 7 days prior to fixation; cell phenotype was visualized with fluorescent probes for F-actin and 4',6-diamidino-2-phenylindole (DAPI) and immuolabeled E-Cadherin. Caco-2 cells were seeded onto 6 mm segments of treated bladders and differentiated for assessment of transepithelial electrical resistance (TER).
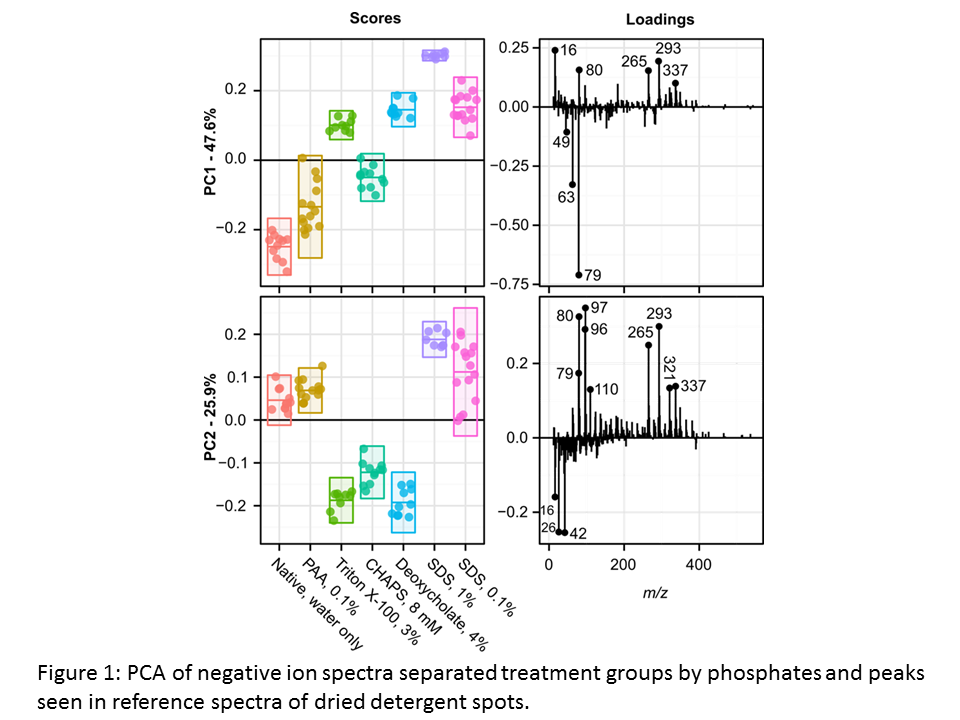
Results and Discussion: Principle component analysis (PCA) of negative ion spectra separated detergent treatments by the presence of phosphates and peaks observed in reference spectra for detergents, previously applied to silicon wafers (Figure 1).
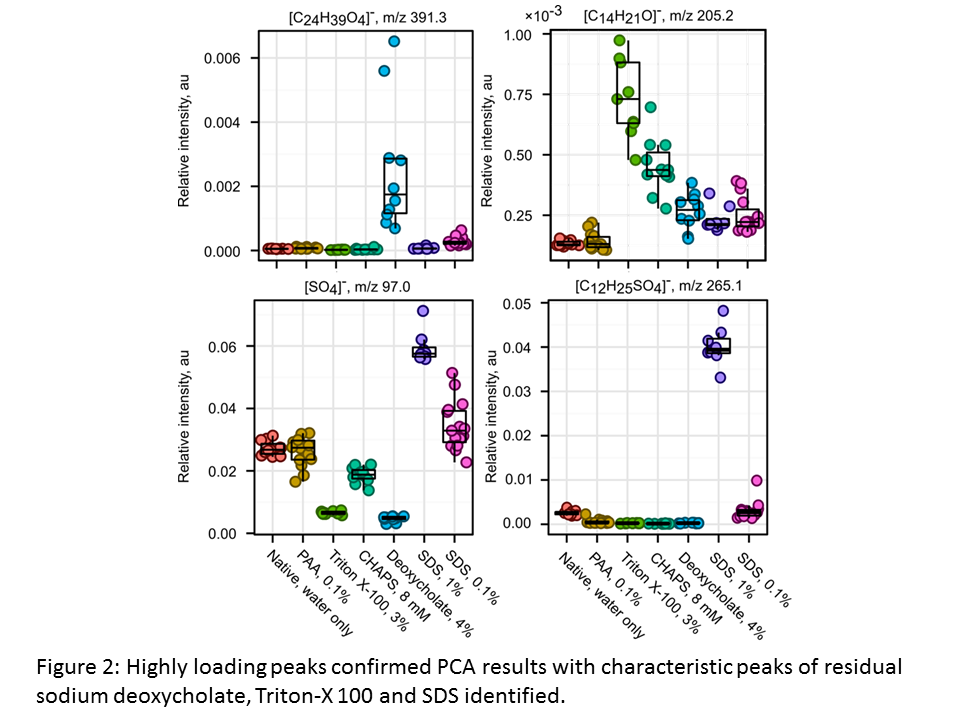
Highly loading peaks confirmed PCA results with characteristic peaks of residual sodium deoxycholate, Triton-X 100 and SDS identified (Figure 2).
Viability, proliferation and phenotype of HUCs, demonstrated by F-actin and DAPI, was maintained on all bladders except those treated with SDS. HUCs cultured on water treated bladders expressed E-Cadherin, localized to intercellular borders in stratified cell layers whereas HUCs cultured on detergent treated bladders showed moderate, diffuse E-Cadherin expression. TER measurements confirmed trends observed with ToF-SIMS and HUC behavior with highest values obtained for water and PAA treated bladders, moderate values with Triton-X 100, sodium deoxycholate and CHAPS treated bladders and lowest TER measurements for SDS treated bladders.
Conclusions: These results demonstrate the suitability of ToF-SIMS to characterize the surface molecular composition of the BMC of porcine urinary bladders treated with detergents commonly used for decellularization. Residual detergent fragments from SDS, Triton-X 100 and sodium deoxycholate were identified in the spectra of the BMCs. Treatment of bladders with 0.1% and 1% SDS affected both the phenotypic characteristics of HUCs and the TER of Caco-2 cells seeded onto the basement membrane complex. An understanding of the effects of detergent exposure on BMC surface molecular functionality will facilitate a rational strategy for successful in vitro and in vivo recellularization techniques.
LJW is funded by an International Outgoing Fellowship (Marie Curie Actions) under the European Union's Seventh Framework Programme (FP7/2007-2013).