Aim: Hydrogels utilized as bioinks for biofabrication have to meet conflicting demands. Whilst 3D printing typically requires high polymer concentrations, low concentrations are generally more suited for cell encapsulation and subsequent 3D cell culture. Therefore, we have developed a hybrid printing technology that combines a strong hydrogel with a bioink to produce reinforced constructs. Specifically, the aim of this study was to evaluate modified poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO) [Poloxamer 407] based gels, for 3D printing of the reinforced bioink structures.
Methods: PEO-PPO-PEO triblock copolymers were chain-extended with α-hydroxy acids: ε-caprolactone, D,L-lactide or an equimolar mixture of L-lactide and glycolide. These were subsequently end-group functionalized by reacting with methacrylic anhydride. Oligoester chain length and hydroxyl conversion to methacrylate groups was analyzed by 1H-NMR. Three-dimensionally printed constructs were generated and for each layer, a meandering grid of a 28.6%wt PEO-PPO-PEO gel was deposited at 21°C. A 5%wt. GelMA bioink at 37°C was subsequently deposited between adjacent strands of reinforcing gel (Figure 1A). CAD models of an auricular implant and a femoral condyle were translated to gcode and printed on a bioprinter (Figure 1B-E). Equine chondrocytes were encapsulated in the bioink at a concentration of 1∙106 cells/ml and viability was measured after one day using live/dead staining. Degradation kinetics were studied for the different oligoester types by submerging cast cross-linked PEO-PPO-PEO disks in PBS at 37°C and measuring the Young’s modulus at selected time points.
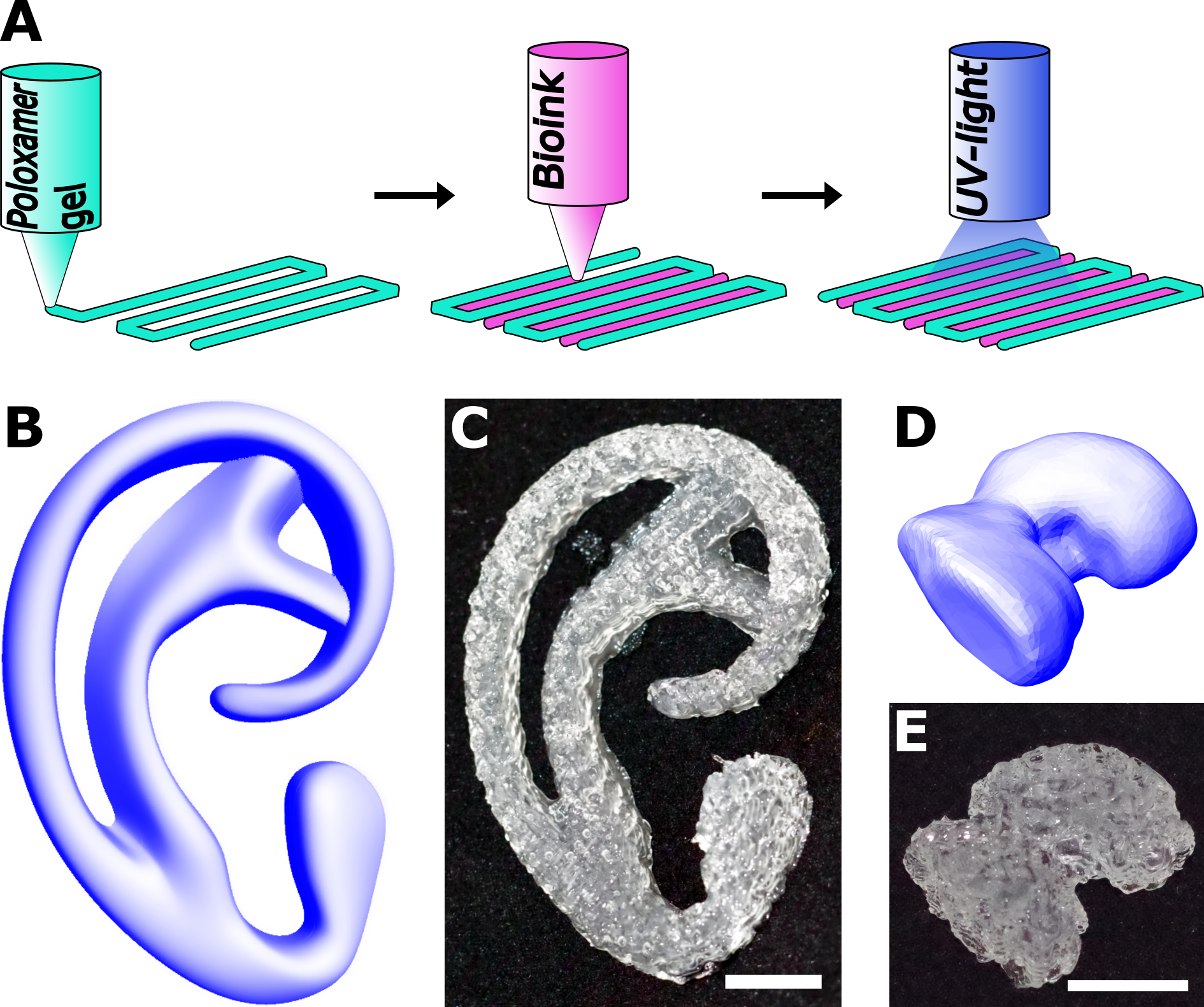
Figure 1: A) Illustration of the hybrid bioprinting approach; B,C) CAD model and 3D printed construct of an auricular implant; D,E) CAD model and 3D printed construct of a miniature femoral condyle. Scalebar = 10 mm.
Results: In the presence of a photo-initiator and light, the resulting macromonomers form hydrolysable, covalently cross-linked polymer networks. Rheological measurements confirm that hydrogels based on modified PEO-PPO-PEO copolymers retain their shear thinning behavior and temperature dependent viscosity. The degradation time could be tailored by selecting different spacers, as gels with a lactide-co-glycolide spacer fully degraded in 2 weeks, while gels with a caprolactone spacer showed limited degradation after a year. Complex anatomical shapes could be printed using the hybrid bioprinting approach. Further, by increasing the spacing between strands of reinforcing gel in biofabricated constructs from 1.35 mm to 2.7 mm, the stiffness of the final construct was raised from 146 to 237 kPa without affecting cell viability.
Conclusions: The hybrid bioprinting technique allows for the creation of complex structures, where mechanical properties and print outcome are mainly governed by the reinforcing gel.
FP7/2007-2013 (n309962-HydroZONES)