Introduction: Tissue engineering has the potential to revolutionize modern medicine and a promising direction that has emerged within tissue engineering scaffold design is the use of polymeric fibers capable of mimicking the natural extracellular matrix (ECM) [1]. However, conventional methods of polymeric fiber fabrications (i.e. electrospinning) suffer from unorganized fiber network formation and difficulty in controlling the uniformity and orientation [2]. Addressing these challenges led us to develop a strategy using a 3D printer and near field electrospinning (NFES) [3] to produce highly aligned 3D fiber structures.
Materials and Methods: A combination of Makerfarm 3D printer (Prusa 8" i3v Kit) and NFES was tailored to function as a 3D printing NFES device. A polymer solution of 18 – 22 % Polycaprolactone (PCL) solution in acetone and acetic acid (80/20) was injected through a 25-gauge needle at a flow rate of ~50 µLh-1 while the stage and nozzle moved in x and y directions (80 cms-1). Next, MC3T3E1 were cultured on top of PCL substrates for 3 days in growth medium at 37ºC and 5% CO2. For cell seeded collagen gel experiments, a basic solution (1 N NaOH) was used to neutralize the acidic collagen solution (3.3 mgml-1, Collagen I, rat tail). Finally, CellProfiler software was used to analyze the morphological parameters of samples that were fixed and labeled using CF568 conjugated phalloidin and DAPI.
Results and Discussion: We successfully spun highly aligned and controlled PCL microfibers (1.88 ± 0.62 µm) on poly(2-hydroxyethyl methacrylate) coated glass surfaces using 3D NFES. We demonstrate simple cell substrate patterns such as a single fiber (basic ECM) as well as more complex cell patterns such as grids and wavy parallel lines (complex ECM) made with 3D NFES (Fig 1).
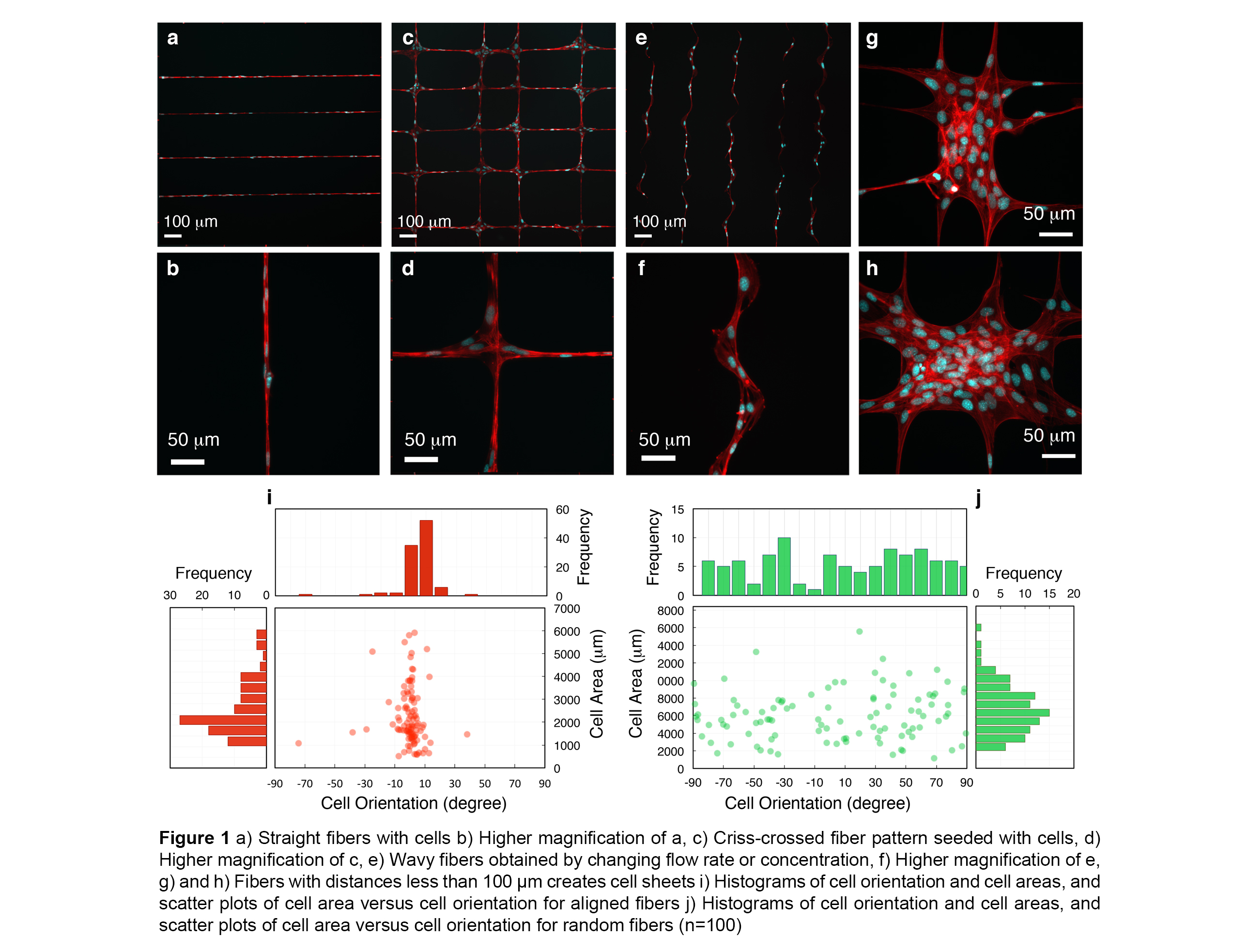
Figures 1i and 1j display scatter plots of fiber nuclei orientation versus cell area and corresponding frequencies. The orientation of cell nuclei demonstrates sensitivity of cells to topographical properties (i.e. aligned cells < 95% of cells lay between ±15°). Finally, we combined a layer of parallel PCL fibers with a cell seeded collagen gel (Fig 2). This combination of collagen and fibers theoretically can improve the mechanical properties of a collagen gel, regulate the anisotropy of the gel mechanics, delay the collagen block deformation and demonstrates the capability of 3D NFES to generate fiber:gel composites.

Conclusions: In summary, we demonstrated a novel technology that uses a combination of two versatile and inexpensive techniques, electrospinning and 3D printing, to produce highly controlled polymeric fibers. The research strategy offers four significant advantages: 1) providing a precision over a variety of fiber patterns over a large area, 2) giving the combination of all three directions of x, y and z for printing fibers, 3) offering an inexpensive method with easy control over shape and orientation, and 4) easy to combine with other pre-patterned biomaterials such as gels.
References:
[1] R. L. Dahlin, F. K. Kasper, and A. G. Mikos, “Polymeric Nanofibers in Tissue Engineering,” Tissue Eng. Part B Rev., vol. 17, no. 5, pp. 349–364, 2011.
[2] W. E. Teo and S. Ramakrishna, “A review on electrospinning design and nanofibre assemblies.,” Nanotechnology, vol. 17, pp. R89–R106, 2006.
[3] D. Sun, C. Chang, S. Li, and L. Lin, “Near-field electrospinning,” Nano Lett., vol. 6, pp. 839–842, 2006.