Introduction: Corneal injury or desease is one of the leading causes of blindness in the world. Due to the limited availability of donor corneal tissue, there is a need for bioengineered corneal implants. Collagen based vitrigel’ biomaterials, with unique biomimetic collagen fibrillar ultrastructure and alignment were developed and characterized, for application as regenerative implants for corneal repair. Molecular interactions between collagen and cyclodextrins (CD), a family of cyclic oligosachharides, allow a unique multilayered self assembly process to maintain implant transparency at biologically relevant thicknesses. Further, functionalized cyclodextrins can be used to modulate vitrigel membrane properties.
Materials and Methods: Vitrigels were fabricated using collagen type I (Col) as described previously[1]. Cyclodextrins (α-, β-, and γ- CD) were incorporated into the membranes during preparation. In addition, vitrigels with modified CDs with different functional groups including methyl, thiol, carboxylic acid, phosphate, were also evaluated. Light transmission of the membranes was measured in a Synergy 2 microplate reader (Biotek). Vitrigel samples were processed for Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy ((TEM) and stained with uranyl acetate; images were taken in a LeoFESEM and Philips CM120 TEM respectively. Rheology was performed on the materials using ARES G2 (TA Instruments).
Results: CD-incorporated collagen (CD-Col) implants displayed clinically relevant levels of transparency. Light transmission over visible light range (Figure 1) was significantly higher in CD-Col membranes than vitrigels without CD.
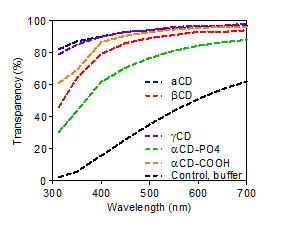
Figure 1: Light transmission of vitrigel materials is vastly improved when CDs are incorporated
Collagen fiber alignment in the implant was exhibited using SEM (Figure 2a) and TEM imaging (Figure 2b). Certain functional groups on CD-Col vitrigels demonstrated unique self-assembly wherein a lamellar collagen ultrastructure, similar to native cornea, is obtained. Cross-sectional images allow visualization of cornea-mimetic layered structure through SEM and TEM (Figure 2c, 2d respectively).
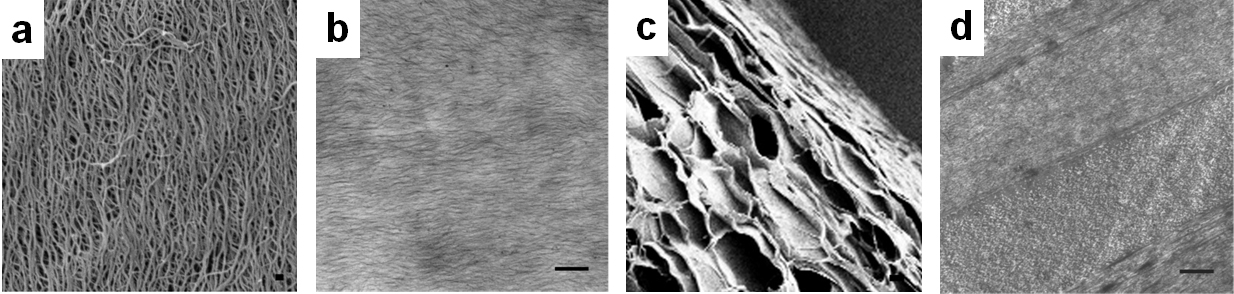
Figure 2: Alignment of collagen fibers visible through a) SEM and b) TEM imaging. Cross-sectional images depict lamellar structure through c) SEM and d) TEM imaging.
This effect is also observed in macro-scale, as the lamellae can be carefully separated using forceps. Rheological measurements show increased storage moduli for CD incorporated materials over a range of oscillation frequencies (Figure 3). Storage, loss moduli and complex viscocity of alphaCD-Col is shown in Figure 3b.
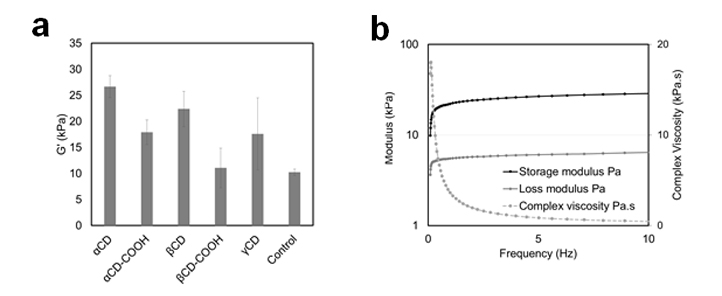
Figure 3: a) Comparison of storage modulus of different CD Col formulations. b) Storage and loss moduli wiht complex viscosity of αCD Col
Conclusions: Cyclodextrins can interact with collagen molecules, and exhibit potential to control the organization of collagen fibrils and influence higher order self-assembly processes. Cornea mimetic ultrastructure of the implants also correlate with greatly increased transparency and improved structural integrity observed through rheology. Furthermore, this study shines light emphasizes the impact of small molecule interaction in the collagen assembly process.
References:
[1] Q. Guo, et al, "Modulation of keratocyte phenotype by collagen fibril nanoarchitecture in membranes for corneal repair", Biomaterials Dec 2013;34(37):9365-72.