Introduction: Each year, nearly hundred thousand patients undergo peripheral nerve surgeries in Europe and U.S[1]. Although there are ample microsurgery techniques available for short nerve lesions, our ability to bridge long peripheral nerve gaps remains unsatisfactory[2]. Current approaches focus on enhancing axon growth by direct action on nerves, or glial cells, but here we investigate a novel approach to influencing regenerative outcomes by biasing the inflammatory sequence early after the injury[3],[4].
After an inflammatory insult, macrophages that accumulate at the site of injury appear to be largely derived from circulating monocytes[5],[6]. In rat, monocytes include two major subsets (CX3CR1 low and high) which appear to be specialized for different functions, but also may be predisposed to differentiate into macrophages of different phenotypes (M1 or M2 macrophages)[5]-[7]. These different subtypes of monocytes can be recruited by distinct cues into inflamed tissues[8], and thus, therapies that target them few hours after the injury may lead to favorable results of resolution of lingering inflammation and subsecuently enhanced growth-perimisive environemnt. Here, we hypothesized that preferential recruitment of anti-inflammatory reparative monocytes (CX3CR1hi) early after the injury via local delivery of Fractalkine (CX3CL1) within the lumen of nerve conduit should result in a better neural repair.
Materials and Methods: Polysulfone tubes filled with Agarose mixed with 10 mg/ml rat recombinant chemokine Fractalkine or IL-4 (n=12). Scaffolds were implanted in adult Lewis male rats according to the method described previously[4]. Four weeks post implantation, scaffolds were explanted for histological analysis of nerve regeneration and macrophage phenotyping. For monocyte depletion, a suspension of clodronate liposome was intravenously injected (0.1ml/10gr), 48 hours before the implantation.
Results and Discussion: Here, we used IL-4 as our benchmark condition, since we have already established the superiority of its regenerative effect in previous studies[4],[9]. However, as shown in Fig.1, the number of regenerated axons at the distal end of the Fractalkine scaffold is dramatically higher than IL-4 scaffold.
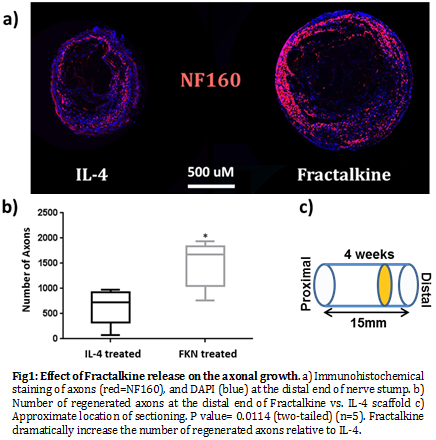
Also, although the number of macrophages at the distal end of the nerve conduit is significantly lower in the Fractalkine scaffold, the ratio of pro-healing macrophages (M2a/M2c) to the total macrophages is significantly higher relative to the IL-4 scaffold (Fig.2). The results of our Clod-lip (monocyte depletion) experiment abrogated the gains in regeneration, clearly demonstrating the central role of monocyte/macrophages in enabling Fractalkine regenerative effect (Fig.3).
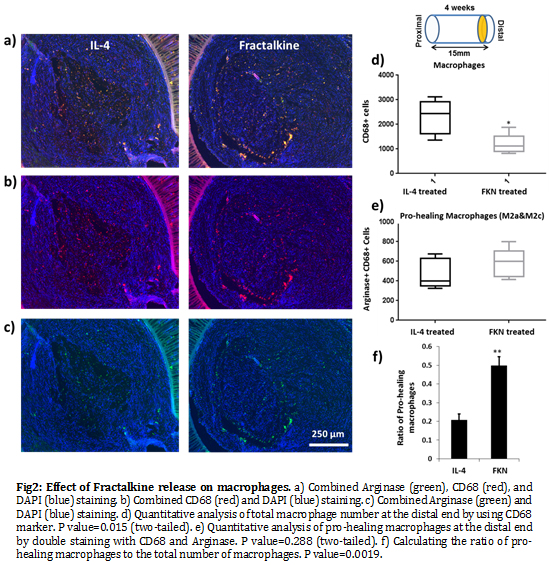
It has been shown that the signal supporting the M2 macrophage environment is produced immediately after healable axotomy and appears to be present within the nerve, well before monocytes infiltration[10]. Therefore, it is coherent that mimicking the axotomy immune response (anti-inflammatory) in the synthetic nerve conduit by early release of exogenous Fractalkine is an effective way to create a permissive environment for neural regeneration. Moreover, the correlation between the phenotype of macrophages and nerve repair outcome was reconfirmed.

Conclusion: Here, we demonstrated that early exposure to Fractalkine after injury can result in dramatic axonal growth, enabled by monocyte/macrophages induced permissiveness. Therefore, monocytes could represent an upstream ‘lever’ to influence downstream fate of peripheral nerve regeneration.
Funding from NIH (NINDS) 1R01 NS093666 (to RVB) is gratefully acknowledged.
References:
[1] Schlosshauer, B., Dreesmann, L., Schaller, H.-E. & Sinis, N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery 59, 740–7; discussion 747–8 (2006)
[2] Bellamkonda, R. V. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials 27, 3515–8 (2006).
[3] Mokarram, N. & Bellamkonda, R. V. Overcoming endogenous constraints on neuronal regeneration. IEEE Trans. Biomed. Eng. 58, 1900–6 (2011
[4] Mokarram, N., Merchant, A., Mukhatyar, V., Patel, G. & Bellamkonda, R. V. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33, 8793–801 (2012).
[5] Cortez-Retamozo, V., Etzrodt, M. & Pittet, M. J. Regulation of macrophage and dendritic cell responses by their lineage precursors. J. Innate Immun. 4, 411–23 (2012).
[6] Yona, S. & Jung, S. Monocytes: subsets, origins, fates and functions. Curr. Opin. Hematol. 17, 53–9 (2010)
[7] Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–47 (2007).
[8] Awojoodu, A. O. et al. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl. Acad. Sci. U. S. A. 110, 13785–90 (2013)
[9] Mokarram, N. & Bellamkonda, R. V. A perspective on immunomodulation and tissue repair. Ann. Biomed. Eng. 42, 338–51 (2014)
[10] Ydens, E. et al. Acute injury in the peripheral nervous system triggers an alternative macrophage response. J. Neuroinflammation 9, 176 (2012)