Introduction: Aggregates of cells, also known as multicellular aggregates (MCAs), have been used as microscale tissues in the fields of cancer biology, regenerative medicine, and developmental biology for many decades. However, small MCAs (fewer than 100 cells per aggregate) have remained challenging to manufacture in large quantities at high uniformity. Forced aggregation into microwells offers a promising solution for forming consistent aggregates, but commercial sources of microwells are expensive, complicated to manufacture, or lack the surface packing densities that would significantly improve MCA production. To address these concerns, we custom-modified a commercial CO2 laser cutter to provide complete control over laser ablation and directly generate microwells in a poly(dimethylsiloxane) (PDMS) substrate.
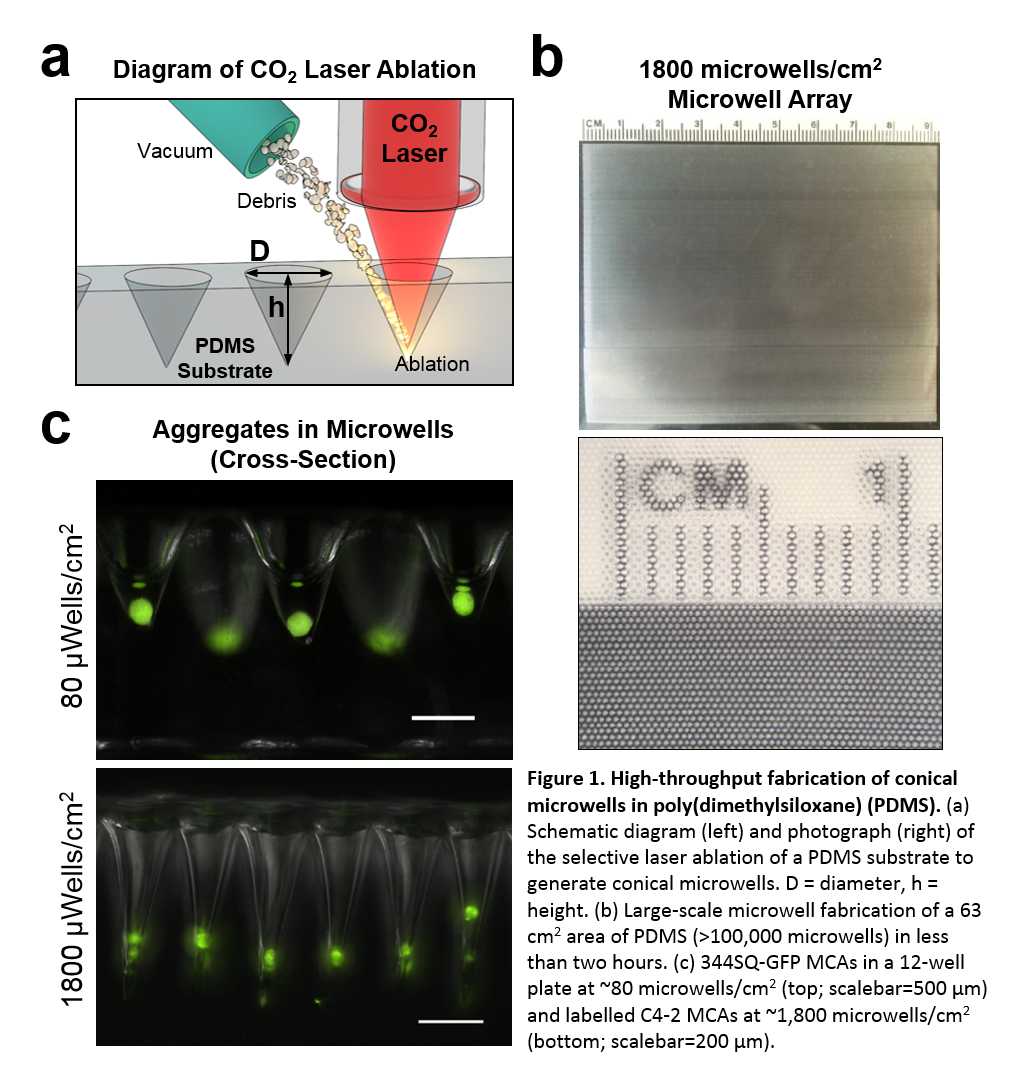
Materials and Methods: We demonstrate a cost-effective CO2 laser ablation system for fabricating microwells in poly(dimethylsiloxane) (PDMS). We achieved this by modifying a relatively inexpensive CO2 laser cutter with an open-source 3D printing microcontroller workflow, a z-axis stage, and a vacuum to prevent ablation debris accumulation. Our system produces microwells in the forms of circular inserts for standard multiwell tissue culture plates, which can be seeded with cells for aggregation in a single pipette step. Computer vision software enabled ultra-high-throughput analysis of aggregates harvested from microwells. Casting of high-fidelity microneedle masters in polyurethane allowed for non-ablative microwell reproduction through replica molding, lowering the technical barrier for implementation in non-technical settings.
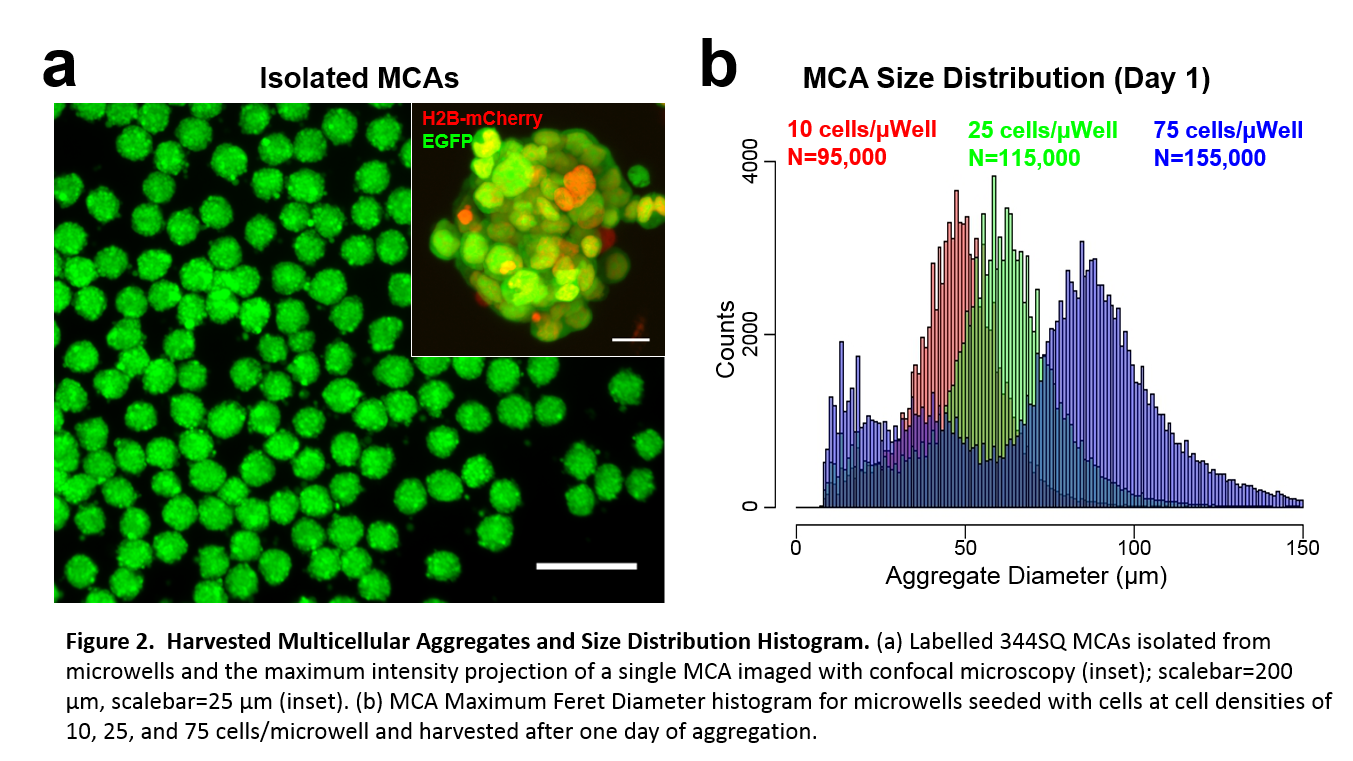
Results and Discussion: Our customized laser cutter setup was capable of ultra-rapid microwell production speeds (>50,000 microwells/hr) at high areal packing densities (1,800 microwells/cm2) over large surface areas for cell culture (60 cm2). Variation of the PDMS substrate distance from the laser focal plane during ablation allowed for the generation of microwells with a variety of sizes, contours, and aspect ratios. MCAs of human bone marrow derived mesenchymal stem cells (hMSCs), murine 344SQ metastatic adenocarcinoma cells, and human C4-2 prostate cancer cells were generated in our system with high uniformity within 24 hours. For 344SQ cells, we generated more than 100,000 MCAs with low diameter polydispersity (62.0 ± 10.8 µm diameter) when seeding at 25 cells/microwell cell density. Moreover, MCAs formed in our microwell system maintained invasive capabilities in 3D migration assays. 344SQ MCAs demonstrated epithelial lumen formation on Matrigel®, and underwent EMT and invasion in the presence of TGF-β.
Conclusion: We have improved fabrication of microwells in PDMS by CO2 laser ablation through incorporation of a 3D printing microcontroller system to control a basic commercial laser cutter. Our improved fabrication method gives us the ability to easily generate multicellular aggregates in massive quantities. Open-source hardware, low cost equipment, and low technical requirements equate to ready implementation in other laboratories. We therefore expect our technique for high-throughput fabrication of customized microwell structures will find broad utility in the generation and cultivation of multicellular aggregates for use in regenerative medicine and tumor engineering applications.
Cancer Prevention Institute (RP120713-P2); National Institute of Health (PO1 CA098912); Joel M. Sederstrom and Cytometry and Cell Sorting Core at Baylor College of Medicine