Introduction: Macrophages are plastic cells that reside in every tissue and participate in a variety of roles in our body. They become activated in response to environmental cues[1]. The three basic functions of macrophages are host defense (classically activated), wound healing and tissue repair, and the general maintenance of tissues (regulatory). Dysregulation of these phenotypes is implicated in a wide range of diseases (osteo- and rheumatoid arthritis, cancer, and diabetes), and contribute to preventing regeneration after injury[1]-[3].
Bioelectric signals such as cell resting transmembrane potential (Vmem) have been shown to be involved not only in the regulation of tissue patterning at the scale of the organ, but also in the control of behavior at the scale of the cell[4]. Specifically, at the level of the cell, Vmem can control cell proliferation, migration, and differentiation (Figure 1A)[5]. A depolarized membrane potential (less negative) is associated with proliferative cells such as stem cells whereas a hyperpolarized membrane potential (more negative) is associated with differentiated/specialized cells such as neurons.

This work aims to establish a relationship between macrophage phenotype and Vmem (Figure 1B). If successful, this concept can then be applied to designing materials to intelligently modulate macrophage membrane potential to promote regeneration.
Methods: A 3D cell model with the mouse macrophage cell line, Raw 264.7, encapsulated at 5x106 cells per mL in poly(ethylene glycol) diacrylate hydrogels was used to study the ability of membrane potential to control macrophage phenotype. Experimental groups consisted of: 1) classically activated macrophages and 2) classically activated macrophages with depolarized Vmem. Raw 264.7 cells were classically activated with lipopolysaccharide (LPS) throughout the study. To depolarize the Vmem of the cells, 80 mM potassium gluconate was added to media of selected gels. After 5 days of culture with daily media changes, western blots were conducted for the following markers of macrophage phenotype: 1) classical activation - TNF-α, IL-1β, and IL-12β, 2) wound healing - Arg-1 and VEGF, and 3) regulatory - IL-10. Western blots were quantified and normalized to DNA levels.
Results and Discussion: Classically activated macrophages with a depolarized Vmem produced substantially lower levels of pro-inflammatory markers (Figure 2A). This effect appeared to be particuarly marked for TNF-α and IL-1β. Furthermore, depolarization resulted in an increase in the regulatory marker IL-10 and the wound healing marker Arg-1 (Figure 2B). The expression of the wound healing marker VEGF also appeared to be reduced in depolarized macrophages, although this difference was not significant (Figure 2B).
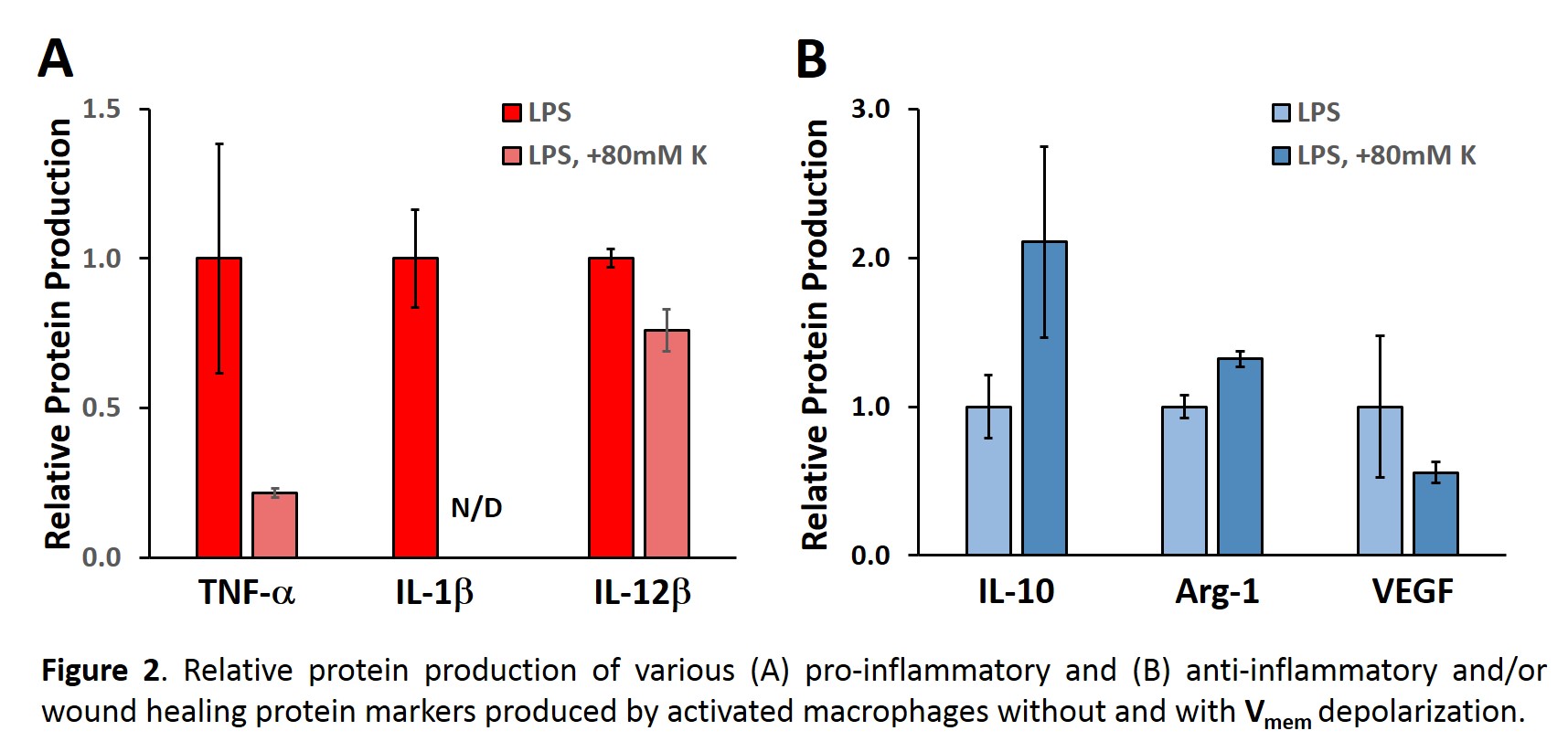
Conclusions: Classically activated macrophages appeared to transition from a pro-inflammatory to a regulatory or wound healing phenotype (anti-inflammatory) following membrane depolarization. These shifts in macrophage phenotype following depolarization occured despite continued macrophage stimulation with LPS. Immediate future work will investigate the duration of the beneficial effects of membrane depolarization. In addition, the ability of membrane hyperpolarization to modulate macrophage phenotype will be probed. Longer term future work will focus on incorporating Vmem control mehcanisms into biomaterials to promote regeneration.
NIH R01 DC013508
References:
[1] Mosser DM. Nat. Rev. Immunol. 2008:8:958-69.
[2] Kigerl KA. J Neurosci. 2009:29:13435-44.
[3] Bondeson J. Current drug targets. 2010:11:576-85.
[4] Levin M. Annu Rev Biomed Eng. 2012:14:295-323.
[5] Sundelacruz S. Stem Cell Rev. 2009:5:231-46.