Introduction: Severe skeletal muscle injuries are difficult to heal and can impair muscle function. Common treatments for these wounds such as muscle flaps and stem cell injections are not predictably. One approach to repair muscle is to implant a biomaterial that promotes muscle regeneration and improves muscle function. In this study, we developed a decellularized muscle matrix (DMM) to take advantage of the biophysical and chemical cues naturally available in muscle, and tested DMM’s ability to enhance muscle function and regeneration.
Methods: DMM was harvested from 8-12 week male Sprague Dawley rats, frozen at -80oC, and shipped to Musculoskeletal Transplant Foundation to be decellularized. DMMs were shipped back frozen to Virginia Commonwealth University and kept frozen at -80oC until implanted. For the implants, male Sprague Dawley rats, 8-12 weeks, were anesthetized. The skin flap, fascia, and femoris covering the gastrocnemius were retracted and a central skeletal muscle defect was created by resecting 1x1cm or 1.5x1cm muscle tissue, taking care to preserve the tibial nerve. Sham (positive control), autograft, DMM, and type I collagen sponge (Resorbable Collagen PLUG, Ace Surgical Supply, negative control) were secured to existing healthy muscle. The contralateral side contained no defect. Animal gait, in situ muscle force testing, H&E, Mason’s trichrome, and immunostaining for Pax7, nicotinic acetylcholine receptor-γ and –ε (NAChR-γ and NAChR-ε), and fetal myosin heavy chain (fMyHC) were used to determine muscle function, regeneration, and innervation.
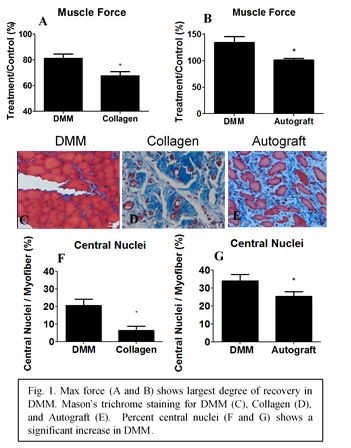
RESULTS AND DISCUSSION: In this study, we demonstrated overall improvements in muscle regeneration in DMM-treated defects during a period of 56 days. Animal gait for DMM and autograft was similar to Sham at 14 days, while collagen-treated sites remained impaired. This suggested a return to normal muscle function with DMM. Muscle force production and force-time curves significantly improved for animals with DMM versus collagen (Fig 1A) or autograft (Fig 1B). Histological staining and immunostaining confirmed functional improvements observed in DMM sites (Fig 1C). Small diameter myofibers with central nuclei were more prevalent (Fig 1F and 1G), and DMM sites exhibited reduced fibrosis compared to autograft. DMM treated sites demonstrated fMyHC and Pax7+ staining; these proteins not only indicate new muscle, but also suggest that fetal NAChR-γ receptors might be present. We demonstrated positive staining for NAChR-γ in DMM and not its mature receptor isoform, NAChR-ε. Autograft stained positive for lower levels of both NAChR-γ and NAChR-ε, neither receptor was present in collagen, and only NAChR-ε was present in Sham. Taken together, these results indicated that DMM mediated improved muscle regeneration and muscle function compared to autograft and collagen.
Conclusion: This research indicated that decellularized muscle can be used to promote repair of large muscle defects. The results also show that it is important to assess treatment effectiveness using multiple outcome measures, including indicators of cell response and muscle function.