Introduction: Osteoclasts (OCs), the bone resorbing cells, are important in the process of bone remodeling. Maintaining bone homeostasis, and balanced control over the bone formation and resorption, require a better understanding of cellular and molecular pathways of OCs function.
Microfluidic platforms emerge as a remarkable tool to decipher, at microscale, the influence of controlled microenvironments on OCs activity.
Our aim was to establish the culture of OCs in compartmentalized microfluidic devices (CMD) and upgrade the system by providing a bone-like mineralized substrates.
Materials and Methods: OCs culture in CMD: OCs were cultured in CMD at different cell densities. The volume of cell suspension was optimized to improve the cell confinement in the microfluidic channel. OCs formation was evaluated through tartrate-resistant acid phosphatase (TRAP) staining and morphological analysis through actin staining.
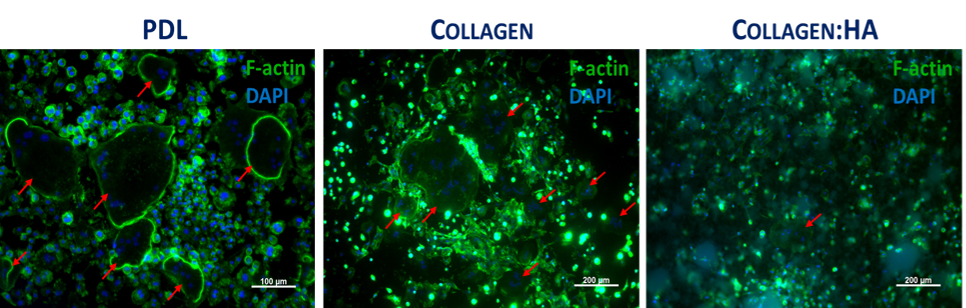
Extracellular matrix (ECM): Collagen was used to coat CMD to mimic the organic component of bone ECM. OCs precursors were seeded upon collagen or poly-D-lysine (PDL - oftenly used for CMD coating). In order to upgrade the system, and provide a mineralized substrate to enhance OCs activity, substrates were prepared from a mixture of collagen with hydroxyapatite (Col:HA, in a proportion of 30:70 (w/w)). The mixture was pipetted to the microfluidic channels prior the seeding of OCs precursors. Cell morphology was observed as stated above.
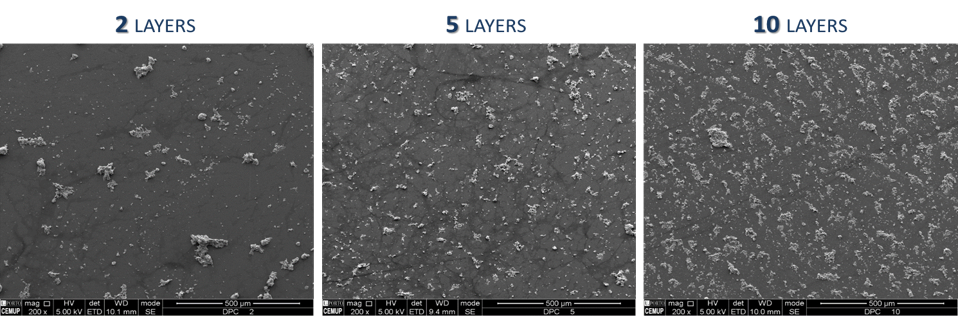
Improvement of mineralized substrates stability: Col:HA solution was prepared in a 30:70 (w/w) proportion to coat glass coverslips with the spin coater. To obtain homogeneous substrate distribution, acceleration and velocity steps were defined, and three different number of coating layers were tested. Afterward, coverslips were incubated with crosslinking agent and dehydrated. The homogeneity of the substrates was observed with scanning electron microscopy (SEM).
Results: OCs were successfully cultured in the CMD. Actin-nuclei staining was performed at 7, 14 and 21 days of differentiation. The cell density, which provided the best yield of differentiated OCs, was 30000 cells/channel. At day 7, multinucleated cells with 2-10 nuclei were detected. From day 14 onwards, multinucleated cells with 20-40 nuclei were observed. TRAP staining confirmed the presence of OCs in the CMD.
To improve the cell adhesion, OCs were cultured on PDL and Collagen coatings. Similar results were obtained for both coatings, confirming the OCs ability to differentiate. Still, the resorption capacity could not be evaluated in these conditions. Thus, the microsystem was upgraded by incorporating a bone-like mineralized substrate (Col:HA) in the CMD.
The Col:HA substrate showed to be degraded at a high rate. To enhance its stability, the spin coating technique followed by crosslinking, was conducted. The time of spinning, acceleration, rotation speed and the layers number were optimized. The optimal values were 1 min, 500 rpm/sec and 9000 rpm, respectively. After SEM analysis, it was verified that the homogeneity of HA distribution increased with the number of layers. The 10-layer coating provided the best outcome.
Conclusion: The culture OCs in the CMD were established. Cell density and coatings to obtain a high yield of OCs differentiation were optimized. The maturation of OCs was improved with the introduction of mineralized substrates. The presented system will refine the readout of in vitro OCs function studies.
This work was financed by Portuguese funds through FCT in the framework of the project PTDC/BIMMED/4041/2014. EN is recipient of PhD fellowship SFRH/BD/81152/2011.