Introduction: We have been using hydrogels of the triblock copolymer of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide), Pluronic F127 (F127) and poly(vinyl alcohol) (PVA) as polymeric biomaterials for the topical release of nitric oxide (NO) from S-nitrosothiols. These systems were already shown to promote skin vasodilation and wound healing [1]. In this work, we show that NO can be released in a exclusively photochemical pathway from S-nitrosoglutathione (GSNO) incorporated in F127/PVA hydrogels and that the rate and total amount of NO-released can be modulated by the F127 and PVA concentrations, widening the possibilities of using these hydrogels for topical NO delivery.
Materials and Methods: GSNO was synthesized through the S-nitrosation of glutathione. F127 solutions were prepared by the cold method. Aqueous PVA solutions were prepared by the previous dissolution of pure PVA at 100ºC under reflux. GSNO was incorporated into the F127 or F127-PVA hydrogels to obtain a final concentration of 100 μM. Real time NO detection was performed using a NO analyzer (NOA 280i, GE Analytical instruments, Boulder, CO, USA) thermostatized at 15°C and 37°C. Irradiation of the solutions and hydrogels was achieved with a visible LED lamp connected to two fiber-optic cables. SAXS measurements were carried out on the SAXS1 beam line at the Brazilian Synchrotron Light Laboratory (LNLS, Campinas, Brazil).
Results and Discussion: The real-time chemiluminescence quantification of NO released from the F127 solutions to the gas phase at 15°C, which is below the critical micelle concentration (CMT) of 19°C for F127 10 wt%, and at 37°C, which is above the CMT of 26°C for F127 20 wt%, showed that the presence of micelles, reduces the rate of photochemical NO release from GSNO . Incorporation of PVA 2.4 wt% in the F127/GSNO solutions led to an aditional reduction in the rates and total amounts of NO released from the hydrogels (Fig. 1). SAXS measurements indicated that the PVA effect on the kinetics of NO release is associated with its action on the packing arrangement of the F127 micelles, which affects the rate of cage-recombination after the homolytic photochemical cleavage of the S-NO bond in GSNO. In addition, NO is expected to concentrate in the hydrophobic PPO nuclei of the micelles, what contributes to a further reduction of its rate of delivery.
Conclusions: Our results show that aqueous F127/PVA/GSNO solutions and hydrogels may allow new forms of modulating the topical NO release, increasing the potential therapeutic applications of these NO-releasing biomaterials.
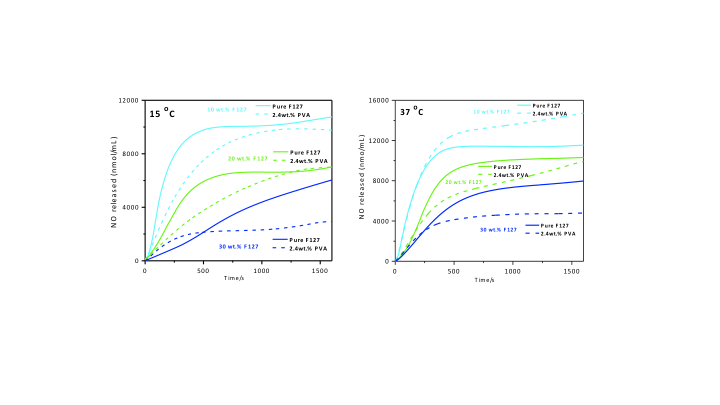
Figure 1. Real time nitric oxide released photochemically form S-nitrosoglutathione (GSNO, 100 μM) incorporated in pure Pluronic F127 hydrogels 10, 20 and 30 wt% in the presence and absence of 2.4 wt% poly(vinyl alcohol) at 15°C and 37°C.
Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP (projects 2013/07173-8; 2014/03266-4)