Use of transwell cell culture and 3D-printing technology to develop an in vitro oviduct model to study bovine fertilization
Marcia
Ferraz1,
Heiko
Henning2,
Kim
Van Dorenmalen3, 4,
Peter
Vos1,
Tom
Stout2,
Ferry
Melchels5,
Jos
Malda2, 3,
Pedro
Costa3, 4,
Richard
Wubbolts6 and
Bart
Gadella1, 6
-
1
Utrecht University, Farm Animal Health, Netherlands
-
2
Utrecht University, Equine Sciences, Netherlands
-
3
Utrecht Medical Center, Orthopedics, Netherlands
-
4
University Medical Center, Utrecht Biofabrication Facility, Netherlands
-
5
Heriot-Watt University, Institute of Biological Chemistry, Biophysics and Bioengineering, United Kingdom
-
6
Utrecht University, Biochemistry and Cell Biology, Netherlands
Introduction: Mammalian fertilization takes place in the oviduct, making it difficult to study in vivo. In vitro models involving cultured bovine oviduct epithelial cells (BOEC) are hampered by a rapid loss of BOEC differentiation properties (e.g. secretory activity and cilia), while BOECs in suspension have a limited lifespan. Here, we report on a tubular BOEC model created by combining Transwell cell culture and 3D-printing technology.
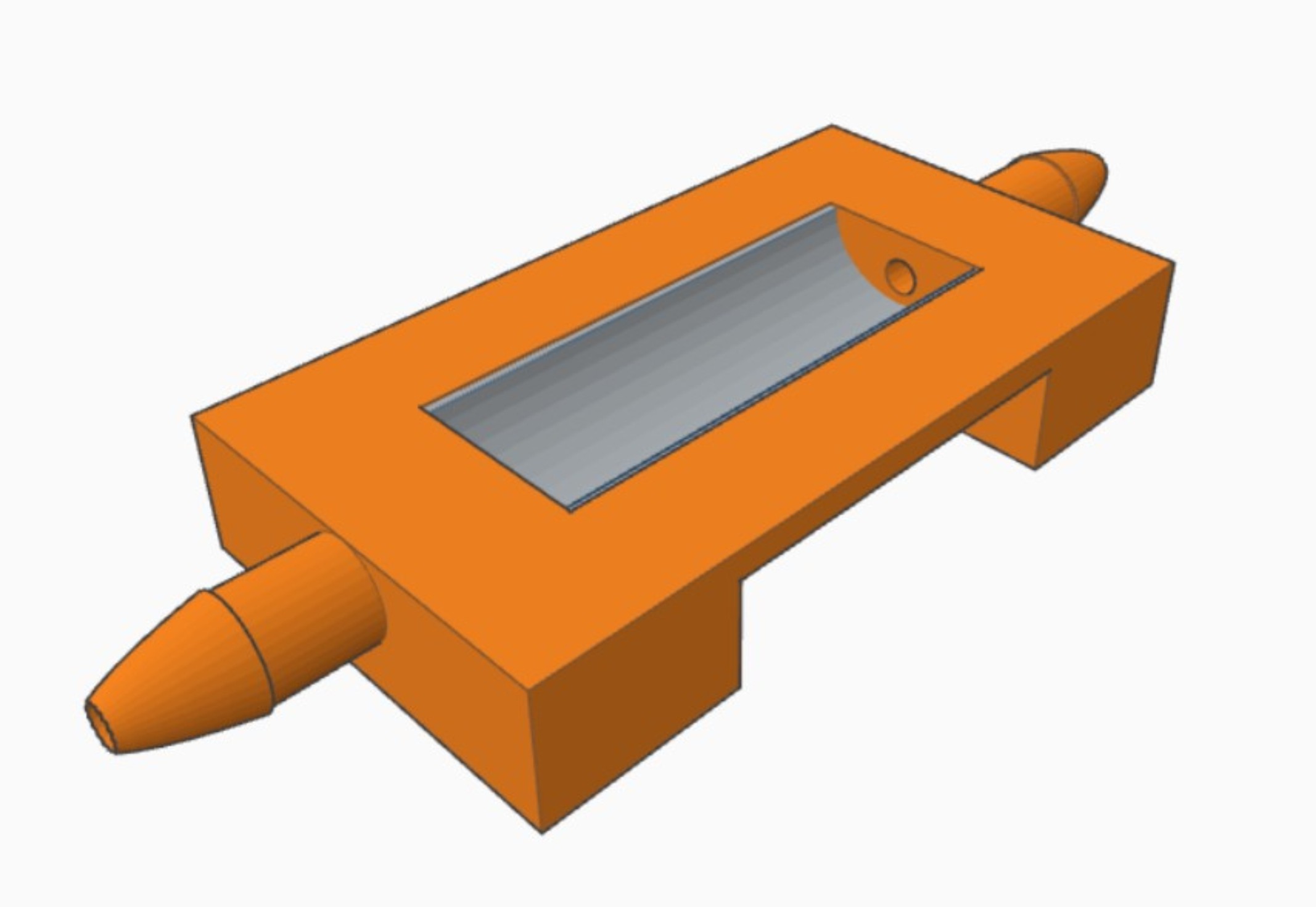
Materials and Methods: U-shape inserts (Figure 1) were 3D-printed using a multi-arm acrylate-based resin (PIC100) on an Envisiontec Perfactory P3 stereolithographer. Post-printing treatments of custom-made tubular inserts were then tested to identify any possible negative impact of the plastics on cell growth. Inserts were either untreated, post-cured with 1000 flashes/side (Otoflash, 66 W), post-cured and Soxhlet-extracted overnight in isopropanol, or post-cured and Soxhlet-extracted over the weekend in water at 37°C. The post-cured and Soxhlet-extracted overnight in isopropanol inserts were the best for culturing BOECs. These inserts were mounted with track-etched PET membranes (12 µm thick, 0.4 µm pore diameter) to create a U-shape. Primary de-differentiated BOEC monolayer cultures (derived from 6 cows) were trypsinized, seeded onto the membranes, grown to confluence (7 days) and allowed to re-differentiate at an air-liquid interface for 28 days. BOECs were also cultured on coverslips as monolayers (2-D culture). Subsequently, the BOECs were (i) fixed and immune labeled to determine their polarized state, or (ii) labeled with HOECHST 33342 and incubated with sperm cells pre-labelled with Mitotracker for live cell imaging analysis.
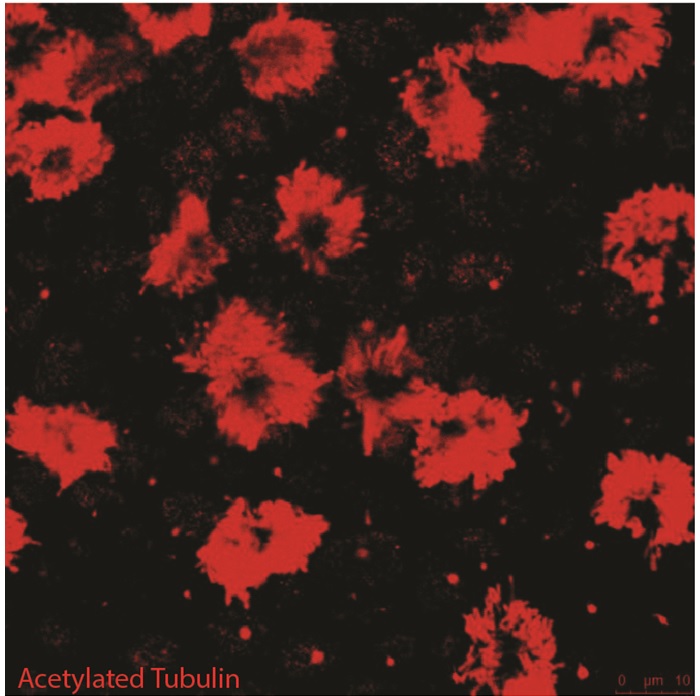
Results and Discussion: Polarization of BOECs (immunodetection of laminin and primary cilia) was evident from day 14 onwards for air-liquid culture but was not detected in 2-D culture. The differentiated status of the cells was indicated by the presence of secondary cilia (immunodetection of acetylated α-tubulin; Figure 2), and started from day 21, with an average of 19 ± 6% ciliated cells; the proportion of ciliated cells increased by day 28 (24 ± 9%); secondary cilia were absent in 2D cultures during the period analyzed. The interaction between sperm and BOECs (in the U-shape insert) could be monitored by live cell imaging using a widefield microscope and LED or laser excitation.
Conclusions: Post-curing and Soxhlet extraction of leachable compounds is crucial to avoid toxic effects on cell growth. The U-shape custom-designed inserts offer a tube-like surface in which BOEC can be cultured to confluency, repolarize and be live cell imaged. Further studies will investigate how to better stimulate ciliogenesis and examine the ability of the in vitro 3D BOEC model to support fertilization in vitro.
Keywords:
Cell Differentiation,
Tissue Engineering,
in vitro,
Imaging method
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Microdevices: reproducing physiology at microscale
Citation:
Ferraz
M,
Henning
H,
Van Dorenmalen
K,
Vos
P,
Stout
T,
Melchels
F,
Malda
J,
Costa
P,
Wubbolts
R and
Gadella
B
(2016). Use of transwell cell culture and 3D-printing technology to develop an in vitro oviduct model to study bovine fertilization.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.00928
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.