Effect of polystyrene and fluoropolymer-based culture vessels on monocyte-based immunotherapy products
-
1
McGill University, Chemical Engineering, Canada
-
2
Saint-Gobain Ceramics & Plastics Inc., Saint-Gobain Fluid Systems, United States
Cell therapy using human donor-derived cells often requires the in vitro expansion or differentiation of cells to generate the cell product of interest. For clinical-scale production, commonly used cell culture vessels manufactured from tissue culture-treated polystyrene, and bag bioreactor systems fabricated from fluoropolymers can be employed. These closed systems have advantages in culture handling and sterility, and have therefore been used for the production of the first FDA-approved, monocyte-based immunotherapy product. Commercially available cell culture surfaces differ in their chemical composition, surface topography, wettability and other physico-chemical properties. These differences may profoundly alter protein adsorption phenomena occurring at the interface of the material and culture medium, and thereby impact cell fate and product performance. The aim of this study was to investigate protein-surface and cell-surface interactions in the context of monocyte-based therapies. To this end, polystyrene and fluoropolymer-based culture surfaces were characterized based on physico-chemical surface properties, protein adsorption from cell culture medium, as well as monocyte adhesion, aggregation and differentiation. By using quartz-crystal microbalance with dissipation monitoring (QCM-D), we found rapid adsorption of a mass of ca. 0.8 μg/cm2 to the fluoropolymer surface (Figure 1, A), which increased its wettability and surface free energy and altered its chemical and mechanical properties. As proteins act as anchors facilitating cell adhesion to surfaces, we hypothesized that the protein layer composition may affect cell-surface interactions. Human monocytes were cultured in polystyrene and fluoropolymer-based vessels and exposed to cytokine cocktails to induce their differentiation and maturation. The expression of surface receptors characteristic for monocyte-derived dendritic cells was assessed by flow cytometry. The cultured cells showed comparable levels of viability, adhesion and aggregation during their maintenance period in the first 2-3 days of culture (Figure 1, B). During the differentiation step, a complete loss of CD14 surface expression was observed, with concomitant upregulation of the antigen-presenting cell-specific surface markers. These results suggest that monocytes can be differentiated into dendritic cells on both fluoropolymer and polystyrene culture surfaces. Differences in cell adhesion kinetics may be related to differences in protein adsorption levels and kinetics previously observed by QCM-D. Understanding cell-surface interactions at a molecular and mechanical level will allow the development of materials tailored for a variety of cell therapy applications.
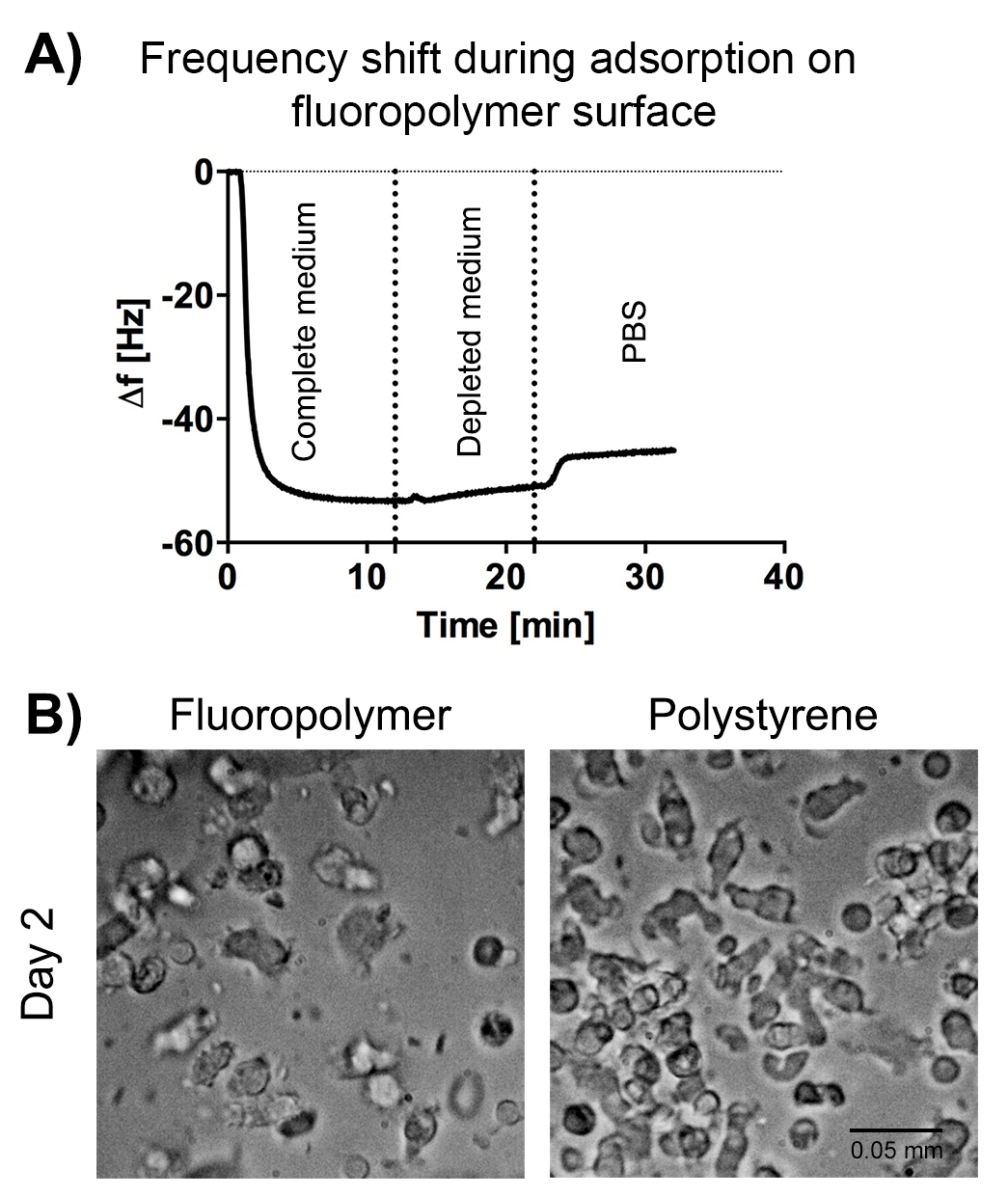
Figure 1. Analysis of protein-surface (A) and cell-surface interactions (B). Fluoropolymers show rapid adsorption of proteins and an adherent cell phenotype similar to polystyrene surfaces.
Saint-Gobain Ceramics & Plastics Inc.; ThéCell network; Centre québécois sur les matériaux fonctionnels (CQMF); Strategic cluster of the Fonds de recherche du Québec – Nature et technologies (FRQNT); Ranjan Roy; Jeffrey Miripol; Natasha Lundgren
Keywords:
cell fate,
surface property,
biomedical application,
Cell interaction
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Biomaterials and cellular signaling
Citation:
Fekete
N,
Wargenau
A,
Xu
S,
Béland
A,
Clark
S,
Tufenkji
N and
Hoesli
C
(2016). Effect of polystyrene and fluoropolymer-based culture vessels on monocyte-based immunotherapy products.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01029
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Natalie Fekete, McGill University, Chemical Engineering, Montreal, QC, Canada, Email1
Dr. Andreas Wargenau, McGill University, Chemical Engineering, Montreal, QC, Canada, andreas.wargenau@mcgill.ca
Dr. Sheng Xu, McGill University, Chemical Engineering, Montreal, QC, Canada, sheng.xu@mail.mcgill.ca
Dr. Ariane Béland, McGill University, Chemical Engineering, Montreal, QC, Canada, ariane.beland@mail.mcgill.ca
Dr. Sarah Clark, Saint-Gobain Ceramics & Plastics Inc., Saint-Gobain Fluid Systems, Northborough, MA, United States, Sarah.L.Clark@saint-gobain.com
Dr. Nathalie Tufenkji, McGill University, Chemical Engineering, Montreal, QC, Canada, nathalie.tufenkji@mcgill.ca
Dr. Corinne Hoesli, McGill University, Chemical Engineering, Montreal, QC, Canada, corinne.hoesli@mcgill.ca