Modulation of the inflammatory response is crucial for the success of implanted biomaterials[1]. Our team has previously shown that incorporation of pro-healing fibrinogen (Fg), in chitosan scaffolds improved bone regeneration and conditioned the systemic immune response[2]. The current study addresses the potential of Fg 3D scaffolds to promote bone regeneration, investigating local and systemic responses to these scaffolds along time after implantation.
Fg scaffolds were prepared by freeze-drying, as previously described for other materials[2], and characterized for morphology and degradation. Cytotoxicity was evaluated according to ISO10993. Animal work was approved by DGAV. Wistar rats (3 months) suffered femoral critical size bone defects, which remained empty or received Fg scaffolds. At 6 days and 8 weeks, animals were sacrificed and blood (BL), lymph nodes (LN), spleen (SP) and femurs were collected. Femurs were analyzed by micro-CT (n=3) and processed for histology (n=5), or used for local gene expression analysis of inflammatory and bone-related markers (n=7). Immune cell populations from BL, LN and SP and their activation status were studied by flow cytometry.
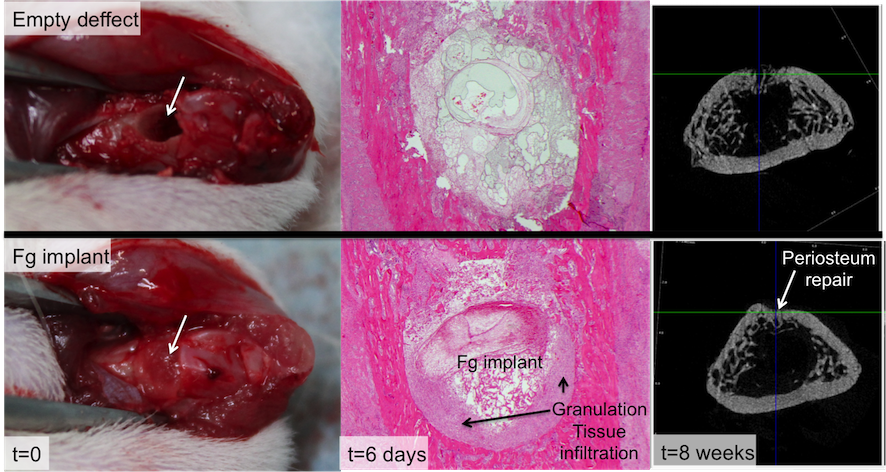
Porous Fg implants were successfully produced, and adopted methodology induced molecular changes in Fg structure. Fg implants were non-cytotoxic and degradation occurred primarily in the first 48h. Histological analysis showed increased infiltration of granulation tissue at periphery of the defect in Fg-group, 6 days upon implantation. This correlated with improved bone regeneration, where Fg scaffolds were replaced by new bone tissue, by 8 weeks post-implantation, as quantified by micro-CT.
Analysis of the immune response at 6 days revealed that at local level response to injury was the most evident, with significant increases in IL-6 and IL-8 mRNA levels for both, empty- and Fg-groups, when compared with non operated animals. However, at systemic level, the Fg group showed significant differences in proportions of immune cells, with significant reduction of B cells, and concomitant increase of T cells, particularly in SP, and reductions in myeloid, NK and NKT cells in BL. Interestingly, Mac-1+ (CD18+/CD11b/c+) cells were significantly decreased in Fg group. Furthermore, a lower percentage of TCRdim T cells and of MHC class II+ CD11b/c+ cells were found in Fg group, indicating lower levels of immune cell activation.
After 8 weeks of implantation, there was still a reduction of B and myeloid cells and an increase in the percentage of T cells in response to Fg implants, but no significant differences were observed between plasma cytokine levels in different groups. Importantly, pro-inflammatory cytokines TNF-a, IL-6 and IL-17a were not detected in plasma, at either time point, indicating low inflammatory response to Fg.
In conclusion, Fg scaffolds promote bone regeneration with complete scaffold integration, which is possibly mediated by modulating the activation status of immune cells.
This work had the financial support of FCT / MEC through National Funds and, when applicable, co-financed by the FEDER through the PT2020 Partnership Agreement under the 4293 Unit I&D. AS, DV, MIO were supported by PhD and post-doc fellowships SFRH/BD/85968/2012, SFRH/BD/87516/2012, SFRH/BPD/37090/2007, respectively.
References:
[1] Mountziaris, PM. et al. Tissue Eng Part B Rev 2011; 17: 393-402
[2] Santos SG. et al. Acta Biomater 2013; 9: 7209-7217