Introduction: Nosocomial urinary tract infections (UTIs) are usually associated with catheterization and are the most common hospital-acquired infection[1]. Previous studies have found that up to 86% of urine specimens of patients undergoing long-term catheterization contained urease-positive bacterial species, of which Proteus mirabilis was the most common[2]. These urease-producing bacteria catalyze the hydrolysis of urea to ammonia, inducing a rise in the pH of the urine[3].
The aim of this study is to exploit the presence of urease-producing bacteria present in most catheter-associated urinary tract infections (CAUTIs) by developing an infection-responsive biomaterial. Surfactant-modified surfaces have been explored for their microbial anti-adherent properties[4]. Here, a surfactant is attached to a polymeric backbone via a linking group susceptible to changes in pH such that an increase in pH will accelerate release of surfactant. This study characterises release of surfactant at pH 7, representing normal physiological urine pH, and pH 10, representing infected urine pH. Release from copolymers is compared to polymer films where the surfactant was physically loaded. The resistance of these materials to bacterial colonization by P. mirabilis and S. aureus is also characterised.
Methods and Materials: Polymeric films were synthesized from mixtures of surfactant, spacer, 2-hydroxyethyl methacrylate, 2,2’-azobisisobutyronitrile and ethyleneglycol-dimethacrylate. Resultant solutions were injected into moulds and polymerized at 60°C for 18 h. Release studies were performed at pH 7 and pH 10 in universal buffer solutions having a constant ionic strength. Bacterial adherence assays using P. mirabilis (Gram-negative) and S. aureus (Gram-positive) were carried out at 4 h and 24 h and were compared to the control material, which contained no surfactant.
Results and Discussion: Figure 1 shows the effect of pH on the release of surfactant from surfactant loaded films and surfactant copolymers at 37°C. Only 56% of the total amount of surfactant is released from the copolymers at pH 7 in comparison to 98% at pH 10. pH would therefore appear to have an affect on the release of surfactant and subsequent diffusion through the polymeric network. pH had no effect on release from physically loaded films, attributable to a lack of ionisable groups in both the surfactant and the polymer.
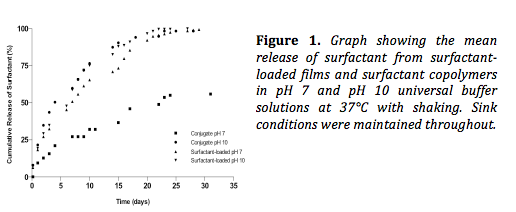
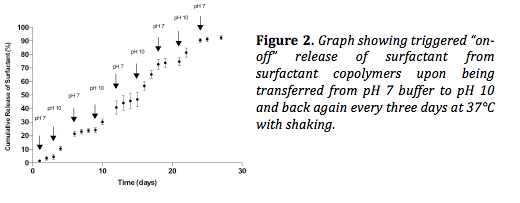
Figure 2 shows the triggered release of surfactant from copolymers upon transferring the copolymer from pH 7 buffer solution to pH 10 every three days. On average, 5 times more surfactant was released during the “on” phase (pH 10) than during the “off” phase (pH 7), demonstrating the ability to trigger release.
With regards to antimicrobial activity, there was a significant reduction in adherence to the materials for both P. mirabilis and S. aureus, in comparison to the control, which contained no surfactant.
Conclusion: These preliminary results are promising with regards to the development of infection-resistant catheter coatings utilizing surfactants in an attempt to bypass issues with antibiotic resistance. The materials demonstrate triggered release as well as encouraging antimicrobial activity against both Gram-negative and Gram-positive bacteria.
Department of Employment and Learning, Northern Ireland
References:
[1] Warren, J.W., 2001. Catheter-associated urinary tract infections. International Journal of Antimicrobial Agents, 17(4), pp.299–303
[2] Mobley, H.L.T. & Warren, J.W., 1987. Urease-positive bacteriuria and obstruction of long-term urinary catheters. Journal of Clinical Microbiology, 25(11), pp.2216–221
[3] Stickler, D.J. & Morgan, S.D., 2006. Modulation of crystalline Proteus mirabilis biofilm development on urinary catheters. Journal of Medical Microbiology, 55(Pt 5), pp.489–494.
[4] Zeraik, A.E. & Nitschke, M., 2010. Biosurfactants as agents to reduce adhesion of pathogenic bacteria to polystyrene surfaces: effect of temperature and hydrophobicity. Current Microbiology, 61(6), pp.554–559.