Introduction: Prevention of bacterial adherence is crucial in reducing medical-device related infection incidence. Current strategies involving incorporation of antimicrobials provide only short-term efficacy due to the finite nature of the active substance loading[1].
Slippery liquid-infused porous surfaces (SLIPS) have recently been introduced as self-cleaning surfaces shown to be effective in prevention of biofouling by marine organisms and bacteria[2]-[5].
Ionic liquids (ILs) are increasingly being used as solvents due to their ‘green’ properties. However, many ILs possess antimicrobial activity that can be easily tuned through careful selection of the anion/cation combination. This work aims to combine the anti-adherent properties of SLIPS with antimicrobial ionic liquids to produce a slippery surface resistant to bacterial infection using medically relevant polymeric materials.
Materials and Methods: Silver-coated PVC samples were synthesised using Tollen’s reagent and a series of synthesised ILs containing the trihexyltetradecyl phosphonium ([P666 14]+), trioctyltetradecyl phosphonium ([P888 14]+) and trioctadecylmethyl phosphonium ([P181818 1]+) cations coupled with a docusate ([AOT]-) anion, were infused to produce SLIPS. ILs were assessed for antimicrobial activity against Staphylococcus aureus (ATCC 6538) and Pseudomonas aeruginosa (PAO1) using minimum inhibitory concentration (MIC) and minimum biocidal concentration (MBC) as per NCCLS guidelines[6].
Anti-adhesive properties of the SLIPS towards these organisms were also determined with an inoculum density of 106 cfu mL-1 at 4 h and 24 h.
Statistical significance was determined using a one-way ANOVA with post-hoc comparisons evaluated using Dunnett’s multiple comparisons test (p < 0.05, n=5).
Results and Discussion: MIC and MBC results for each of the synthesised ionic liquids against S. aureus and P. aeruginosa are shown in Table 1.
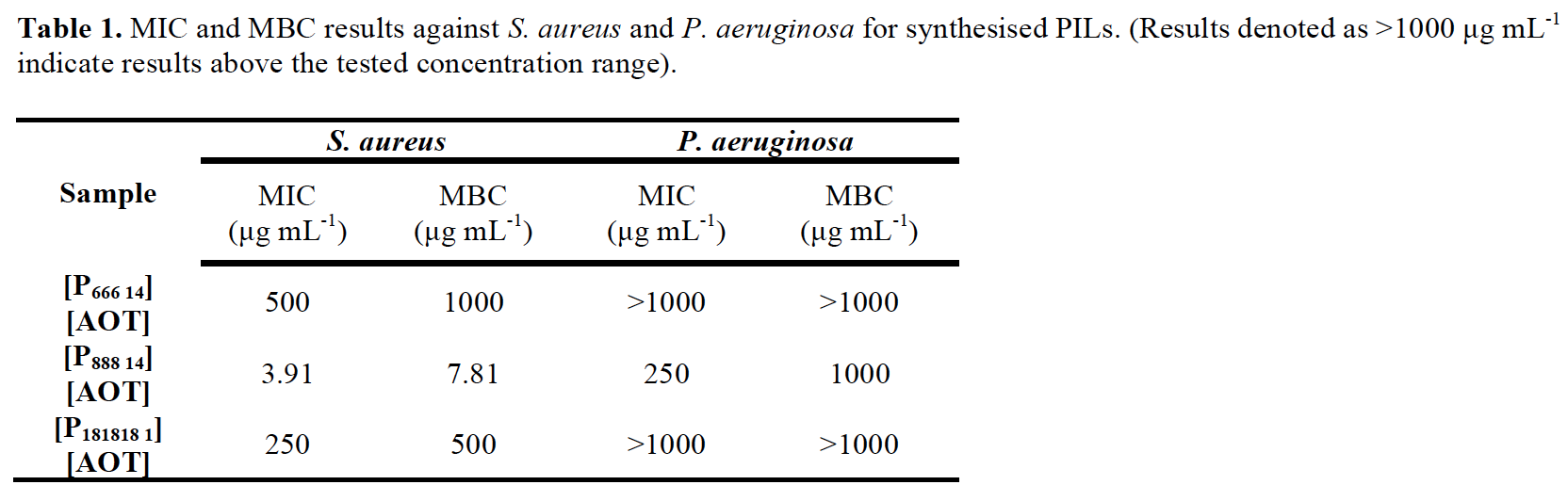
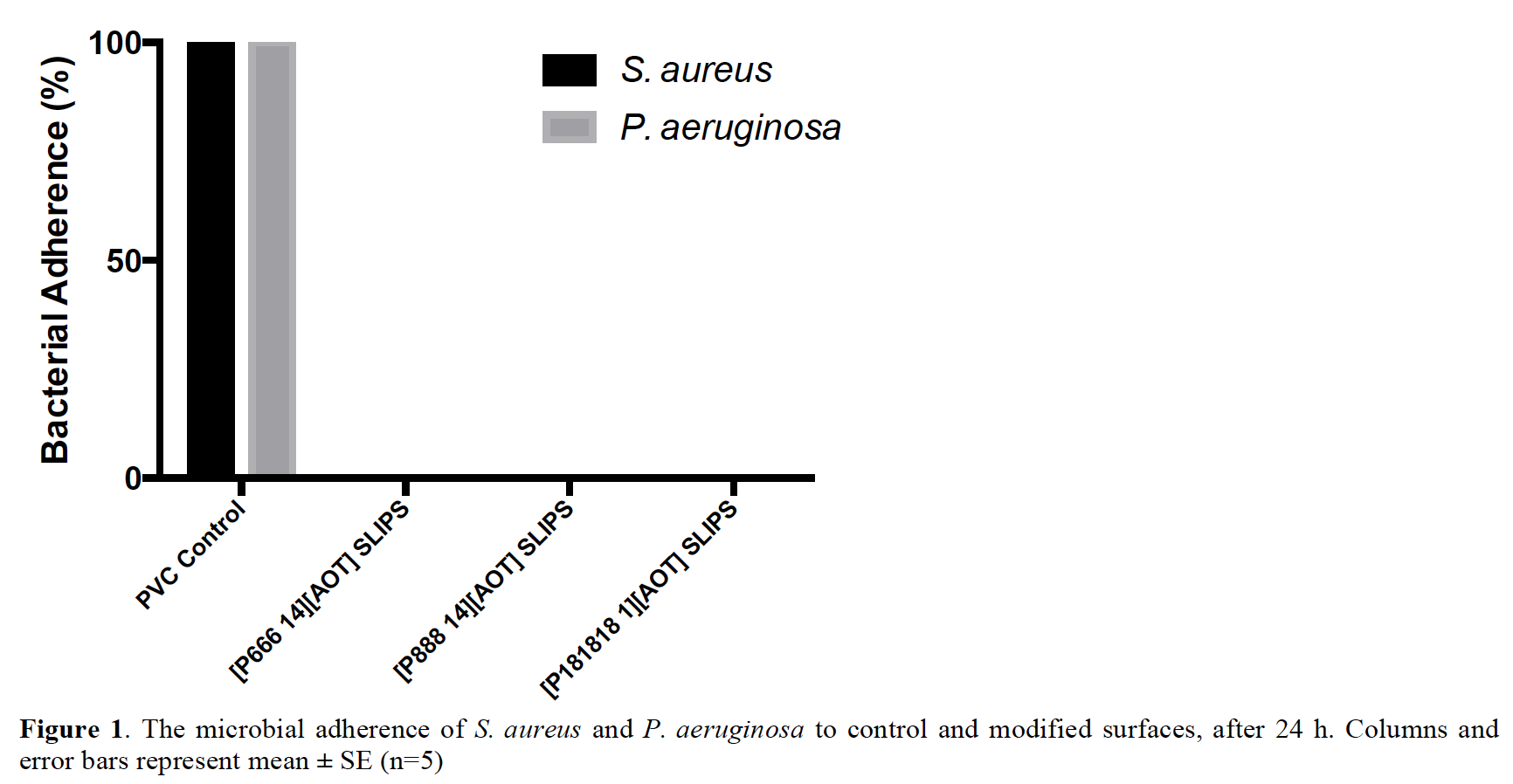
All ILs displayed antimicrobial activity against Gram-positive S. aureus. [P888 14][AOT] possessed the most potent activity against S. aureus as well as moderate activity against Gram-negative P. aeruginosa. Activity against P. aeruginosa was poor and can be explained by its ability to repel hydrophobic solutes such as the ILs tested[7]. The microbial adherence of S. aureus and P. aeruginosa after 24 h to synthesised SLIPS compared to unmodified control uPVC is shown in Figure 1. SLIPS samples displayed complete resistance against microbial adherence by S. aureus and P. aeruginosa after 24 h compared to control. This inhibition of adherence can be explained by the combination of antimicrobial action of the ionic liquids, as well as the loss of attachment sites for bacterial adhesion caused by the formation of a smooth liquid surface created by the SLIPS. This is further confirmed by the assessment of adherence of S. aureus to silver-coated PVC samples which produced only a 1-log reduction in adherence compared to the >5-log reduction produced by the SLIPS materials.
Conclusion: This study has shown the potential of combining the use of antimicrobial ionic liquids with SLIPS to produce an infection-resistant coating for medical devices. Moreover, this can be applied to cheap polymeric materials, such as PVC, commonly used for medical device manufacture.
Department for Employment and Learning - Northern Ireland
References:
[1] Knetsch MLW, Koole LH. New Strategies in the Development of Antimicrobial Coatings: The Example of Increasing Usage of Silver and Silver Nanoparticles. Polymers-Basel. 2011;3(1):340-66.
[2] Epstein AK, Wong TS, Belisle RA, Boggs EM, Aizenberg J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. P Natl Acad Sci USA. 2012;109(33):13182-7.
[3] Li JS, Kleintschek T, Rieder A, Cheng Y, Baumbach T, Obst U, et al. Hydrophobic Liquid-Infused Porous Polymer Surfaces for Antibacterial Applications. Acs Appl Mater Inter. 2013;5(14):6704-11.
[4] Xiao LL, Li JS, Mieszkin S, Di Fino A, Clare AS, Callow ME, et al. Slippery Liquid-Infused Porous Surfaces Showing Marine Antibiofouling Properties. Acs Appl Mater Inter. 2013;5(20):10074-80.
[5] Wong TS, Kang SH, Tang SKY, Smythe EJ, Hatton BD, Grinthal A, et al. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature. 2011;477(7365):443-7.
[6] Carson L, Chau PKW, Earle MJ, Gilea MA, Gilmore BF, Gorman SP, et al. Antibiofilm activities of 1-alkyl-3-methylimidazolium chloride ionic liquids. Green Chem. 2009;11(4):492-7.
[7] Santos AG, Ribeiro BD, Alviano DS, Coelho MAZ. Toxicity of ionic liquids toward microorganisms interesting to the food industry. Rsc Adv. 2014;4(70):37157-63.