Introduction: Supramolecular assembly drives the organization of natural structures—such as the fibrillar structure of collagen in the extracellular matrix. The capacity to harness this organization in synthetic analogues is of interest, including toward development of biomedical materials[1],[2]. In recent years, there have been numerous reports indicating that similar hierarchical structures are attainable in synthetic, supramolecular hydrogels. Though, studies including the effects of polymer concentration and temporal evolution to further understanding of these observations are lacking.
Materials & Methods: To obtain hydrogel precursors for guest-host (GH) assembly, hyaluronic acid (HA) was modified by coupling of 1-adamantane acetic acid (Ad-HA) or aminated β-cyclodextrin (CD-HA) as previously described[3]. Ad-HA was secondarily methacrylated to allow photopolymerization (10mW/cm2, 2mM LAP) to stabilize structures and fluorescent label (with FITC) for imaging. GH hydrogels were formed by dissolution of polymers at the desired concentration, mixing of solutions to afford a 1:1 ratio of guest-host, and swelling in buffer until set time points. Confocal microscopy was used to investigate structure in the hydrated state; measurements (ImageJ) represent mean ± std dev of 10 measurements. For microrheology, 0.2µm beads (carboxyl modified, fluorescent) were entrapped by suspension within the polymer solutions. For tracking, images were acquired at 60 fps with 40x magnification.
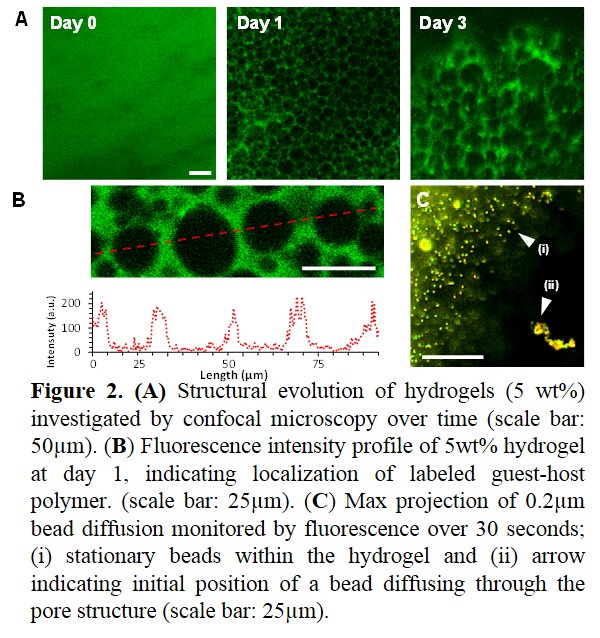
Results & Discussion: Supramolecular guest-host hydrogels were investigated for their ability to drive structural evolution based on hierarchal self-organization (Fig 1A). Low polymer concentrations resulted in particle formation (2.5wt%: D = 6.2±0.9 µm), whereas higher concentrations yielded a porous hydrogels (5wt%: D = 16.6±3.0 µm; 7.5wt%: D = 23.7±5.9 µm). Hydrogels (5 wt%) were initially homogenous, but then developed porous structures by day 1, which evolved over time to increase in diameter with hydrogel swelling and re-organization (Fig 2A). Within these structures, fluorescence intensity profiles (Fig 2B) demonstrate distinct fluorescent signals with greater than 100-fold difference between regions—indicative of a high degree of polymer localization. Further examination via microrheological techniques demonstrated diffusive bead motion within the pore but not the hydrogel phase (Fig 2C), supporting the conclusion of polymer segregation.
Conclusions: Hydrogels formed by guest-host interactions are able to develop structural organization with time. The initial concentration defines the structure developed (particulate or porous structure) while time allows for evolution through dynamic re-organization. These microscale structures have utility toward understanding long-term stability of guest-host hydrogels and their use in biomedical applications.
Financial support provided by a Predoctoral Fellowship (C.B.R.) and Established Investigator Award (J.A.B.) from the American Heart Association.
References:
[1] Mendes AC, et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009.
[2] Wang HY & Heilshorn SC. Adv Mater. 2015.
[3] Rodell CB, et al. Adv Funct Mater. 2015.