Introduction: Human induced pluripotent stem cells (hiPSCs) can generate any type of cell in the body, making them attractive for cell therapy applications. hiPSCs can be directed towards a neural lineage for use in regenerative therapy through delivery of physical and chemical cues[1],[2]. Personalized neural tissue derived from hiPSCs can survive and differentiate into relevant neural phenotypes to replace damaged central nervous system (CNS) tissues and promote functional recovery[4]. The physical microenvironment of regenerating tissues has a significant influence on the behavior of differentiating hiPSCs, therefore such engineered tissues require a 3D biomaterial scaffold with physical properties closely resembling those of the native extracellular matrix found in the CNS[2],[3].
Fibrin, a natural biomaterial involved in blood clotting, has proven to be a suitable scaffold for promoting the generation and viability of neurons from hiPSCs[2],[4]. However, the fast degradation rate of fibrin does not provide an adequate time window to accommodate the regeneration of neural tissue by hiPSCs. Degradation rates of fibrin scaffolds can be decreased by increasing the amount of crosslinking through the addition of a crosslinking agent. Genipin, a plant-derived agent that promotes crosslink formation in fibrin scaffolds, possesses neuritogenic, neurotrophic and neuroprotective properties[5]-[9]. As the addition of genipin alters the physical characteristics of the scaffold, it may also influence the behaviour of the differentiating cells[11]-[13]. This study determines the concentrations of fibrin and genipin that provide the optimal physical properties for the generation of spinal motor neurons from iPSCs.
Materials and Methods: Human iPSCs were cultured in AggreWell™ 800 plates using neural induction medium (NIM) for 5 days to form neural aggregates. These were then seeded in 3D fibrin-genipin scaffolds of genipin concentrations 1 mM, 2.5 mM, 5.0 mM, or 10 mM, with NIM + 1 µM retinoic acid + 1 µM purmorphamine, and cultured for an additional 21 days. Cell viability, adhesion and phenotype were assessed using flow cytometry or immunocytochemistry on days 14 and 21. The elastic modulus and degree of crosslinking for each scaffold was characterized by rheometry and scanning electron microscope.
Results and Discussion: Human iPSCs formed aggregates 24 hours after being placed into the AggreWell™ 800 plates. When seeded into fibrin-genipin scaffolds, neural aggregates adhered and extended neurites. LIVE-DEAD staining showed that neurons were viable, while neural identity was confirmed by immunocytochemistry. The formation of crosslinks due to the addition of genipin to fibrin scaffolds was found to provide superior mechanical stability for the induction of spinal motor neurons from hiPSCs without negatively impacting their differentiation.
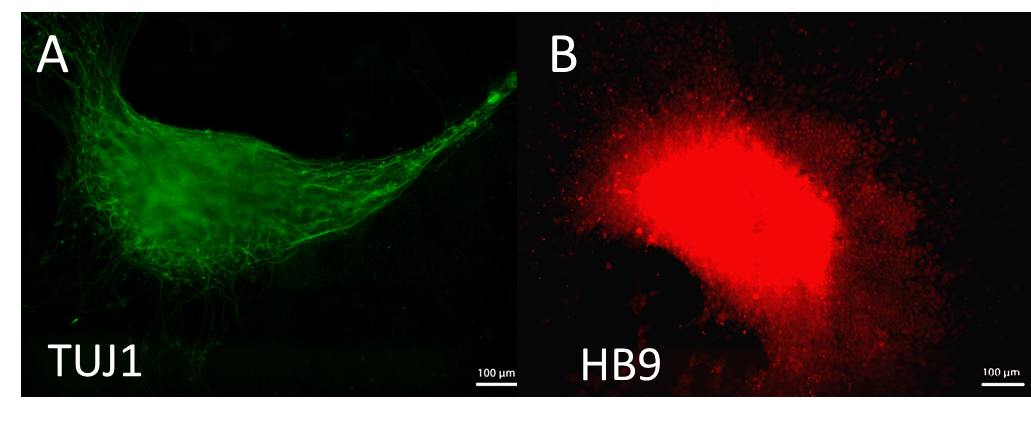
Figure 1. Spinal motor neurons induced from hiPSCs in 3-D fibrin-genipin bioscaffolds, immunostained for A) TUJ1, a neuronal marker, and B) HB9, a marker specific to spinal motor neuron precursors.
Conclusion: The degradation rates of fibrin scaffolds can be tailored to accomodate the induction of spinal motor neurons from hiPSCs by the addition of the crosslinking agent genipin, while maintaining the necessary physical properties to promote differentiation.
The authors would like to thank the Natural Sciences and Engineering Research Council of Canada (NSERC).
References:
[1] Takahashi, K. et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell (2007). doi:10.1016/j.cell.2007.11.019
[2] Montgomery, A., Wong, A., Gabers, N. & Willerth, S. M. Engineering personalized neural tissue by combining induced pluripotent stem cells with fibrin scaffolds. Biomater Sci 3, 401–13 (2015).
[3] Engler, A., Sen, S., Sweeney, L. & Discher, D. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126, (2005).
[4] Lu, P., Woodruff, G., Wang, Y., Graham, L., Hunt, M., Wu, D., ... & Tuszynski, M. H. (2014). Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron, 83(4), 789-796.
[5] Yamazaki, M., & Chiba, K. (2005). Neurotrophic effects of genipin on Neuro2a cells. Journal of health science, 51(6), 687-692.
[6] Yamazaki, M., Chiba, K., & Satoh, K. (2008). Neuro2a cell death induced by 6- hydroxydopamine is attenuated by genipin. Journal of health science, 54(6), 638- 644.
[7] Nam, K. N., Choi, Y. S., Jung, H. J., Park, G. H., Park, J. M., Moon, S. K., ... & Lee, E. H. (2010). Genipin inhibits the inflammatory response of rat brain microglial cells. International immunopharmacology, 10(4), 493-499.
[8] Yamazaki, M., Chiba, K., & Yoshikawa, C. (2009). Genipin suppresses A23187- induced cytotoxicity in neuro2a cells. Biological and Pharmaceutical Bulletin, 32(6), 1043-1046.
[9] Yamazaki, M., Chiba, K., Mohri, T., & Hatanaka, H. (2004). Cyclic GMP- dependent neurite outgrowth by genipin and nerve growth factor in PC12h cells.European journal of pharmacology, 488(1), 35-43.
[10] Schek, R. M., Michalek, A. J., & Iatridis, J. C. (2011). Genipin-crosslinked fibrin hydrogels as a potential adhesive to augment intervertebral disc annulus repair. European cells & materials, 21, 373.
[11] Gamboa‐Martínez, T. C., Luque‐Guillén, V., González‐García, C., Gómez Ribelles, J. L., & Gallego‐Ferrer, G. (2015). Crosslinked fibrin gels for tissue engineering: Two approaches to improve their properties. Journal of Biomedical Materials Research Part A, 103(2), 614-621.
[12] Liu, B. S., Yao, C. H., Hsu, S. H., Yeh, T. S., Chen, Y. S., & Kao, S. T. (2004). A novel use of genipin-fixed gelatin as extracellular matrix for peripheral nerve regeneration. Journal of biomaterials applications, 19(1), 21-34.
[13] McKay, C. A., Pomrenke, R. D., McLane, J. S., Schaub, N. J., DeSimone, E. K., Ligon, L. A., & Gilbert, R. J. (2014). An Injectable, Calcium Responsive Composite Hydrogel for the Treatment of Acute Spinal Cord Injury. ACS applied materials & interfaces, 6(3), 1424-1438.